|
Australasian Biotechnology (backfiles)
AusBiotech
ISSN: 1036-7128
Vol. 11, Num. 2, 2001, pp. 6-9
|
Untitled Document
Australasian Biotechnology, Vol. 11 No. 2, 2001, pp. 6-9
NEWS
Code Number: au01017
$450,000 FOR THE ABA
The Minister for Industry, Science and Resources, Senator Nick Minchin, announced
on 29th March funding of $450,000 to assist the Australian Biotechnology Association
to become a strong national voice for the biotechnology industry.
The need for developing a strong network within the biotechnology industry
was identified under the Commonwealth Government’s National Biotechnology Strategy
(NBS) launched in July last year.
“The Australian Biotechnology Association has provided a valuable focus for
the Australian biotechnology research sector for many years,” Senator Minchin
said. “It is now responding to the needs of our growing biotechnology industry
by developing a more industry-focused organisation.”
The funding, to be delivered over three years, will allow the Australian Biotechnology
Association to expand its focus on representing the interests of the biotechnology
sector. This will include nurturing sustainable biotechnology enterprises; increasing
links between industry and researchers; and increasing awareness of biotechnology
with potential investors, researchers, governments and the broader community.
The Australian Biotechnology Association will develop information databases
such as the Biotechnology Directory and monitor industry trends, allowing the
Association to provide valuable input to Government on policy development.
“I look forward to continuing to work with the Association on building a dynamic
Australian biotechnology industry. This grant of $450,000 will allow the Association
to play a significant role in the development of this key growth sector,” Senator
Minchin concluded.
THE GENOPOLE NETWORK - FRANCE
The genopole at Evry, the first of its kind in France, was set up in October
1998 in the Paris region (Essonne). A ‘technopole’ (a centre for high technology
research and application) specialising in genetics and genomics, it brings together
research laboratories, innovative enterprises and high-level tertiary education.
The way the genopole works facilitates the creation and nurturing of start-ups,
equipping of laboratories and support for research activities. It provides a
variety of common services: communication, international relations, high-speed
IT network connections, a library and so on.
Located around Genethon, a private research institute, the Evry genopole site
benefits from exceptional local resources. Close by are Genoscope, the National
Centre for Genotyping (CNG) and Infobiogen, which have all been set up in response
to the government’s priority program on genomics. The genopole brings together
the principal bodies in French medical research: the National Institute for
Health and Medical Research (INSERM), the National Scientific Research Centre
(CNRS, similar in many ways to the Australian CSIRO) and the Atomic Energy Centre
(CEA), plus the related university research units. On its educational side,
the genopole is linked to the University of Evry-Val d’Essonne. And since its
establishment, the genopole has seen the creation of 12 enterprises, among them
the European leader in genomics, Genset. Firm decisions have also been taken
on a further 17 new enterprises (16 of them start-ups), and 27 more proposals
are being studied.
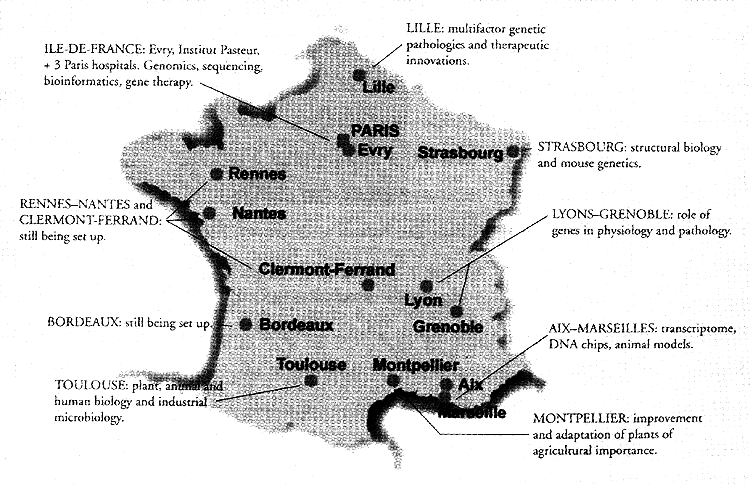
Following the successful development at Evry, other sites for genopoles were
selected in 1999 and 2000: Lille, Strasbourg, Toulouse, Montpellier, Aix-Marseilles,
Lyons-Grenoble and Bordeaux. Each of the eight genopoles has its own program
of research, a high level genetics education program and some effective means
of applying and disseminating its research results. They each specialise in
different areas, to avoid research duplication.
Networking allows the genopoles to coordinate their work and provide mutual
aid. It is a ‘federalising’ program, enabling researchers from different fields
to come together to work on the same project or pursue the same objective in
a way that they would probably never have done otherwise. Moreover, the network
is constructed on local and regional bases (not on a centralised model), enabling
a wider distribution of innovation from the various genopoles. The network of
genopoles offers regional catalysts for public and private research, and serves
as a cradle for new biotechnology firms.
AUSTRALIAN COMPANY PIONEERS APPLICATION OF NON-CODING DNA
GeneType AG, a subsidiary of Australian biotechnology company Genetic Technologies
(GTG - ASX), has applauded the findings of the significance of ‘junk DNA’ (non-coding
sequences) by the Human Genome Project, involving thousands of researchers around
the world.
GeneType is the original pioneer in the field of non-coding sequences. The
company was formed in 1989 specifically to research the significance of so-called
‘junk DNA’. The human genome is made up of approximately 95% ‘junk DNA’.
“GeneType recognised the potential value of non-coding sequences many years
ago while working on human haplotyping. This research proved that the non-coding
‘junk DNA’ region of the human HLA gene complex was in reality not junk, but
in fact a valuable and highly ordered reservoir of useful genetic information,”
said Dr Mervyn Jacobson, Chairman of Genetic Technologies.
“We have also demonstrated that non-coding sequences may be applied to other
areas of the human genome and also to the genomes of animals, plants and even
insects and parasites.”
GeneType demonstrated many uses of these non-coding sequences, in DNA diagnostics,
high-resolution bone marrow typing for transplantation, haplotype identification,
SNP analysis, gene-mapping and in the identification of traits of interest.
“Intron sequence analysis provides a powerful strategy for gene mapping and
exploits GeneType’s unique knowledge of non-coding structures to search for
new genes of special interest, especially disease-causing genes or those associated
with particular traits.
“GeneType currently holds two world-wide patents on the use of intron sequence
analysis (using the non-coding regions of DNA) for particular applications including
gene mapping and haplotyping.
“GeneType has over the years filed broad patents in most countries covering
these applications, and these patents are now issued. Accordingly, from the
comments made over recent days by leading scientists and researchers world-wide,
these is now increasing recognition that to develop their work further they
will need to access the GeneType technology - and must therefore obtain a licence
from us,” Dr Jacobson said.
Genetic Technologies has recently secured patent insurance from GE Reinsurance
Corporation, part of the world-wide General Electrics Group. Now that patent
insurance is in place GTG is proceeding to commence licensing of the patent.
VICTORIA LAUNCHES MAJOR BIOTECH INITIATIVE
The Victorian Government launched on Friday 6th April its new biotechnology
website (www.biotechnology.vic.gov.au
) and its draft Biotechnology Strategic Development Plan for Victoria. The draft
plan of over 70 pages can be accessed via its new website. The plan is comprehensive
and optimistic, with a mission to make Victoria among the top 5 centres in the
world in biotechnology. A large number of actions and action plans are included
in the document, and it seems that most of the agenda is in place, although
the government is at pains to call this a “draft” plan and is calling for comments.
Perhaps this indicates a new positive flexibility in government . Another interesting
document that can be accessed from the same site is consultant David Fayle’s
commissioned article overviewing and updating Victorian-based biotech businesses.
it is also well worth reading to gain a full view of activities in Victoria.
The new government website is very nicely presented and efficient to use. It
complements their innovation website for Science, Technology and Industry (http://www.innovation.vic.gov.au)
GENETIC INFORMATION INQUIRY
The Australian Law Reform Commission (ALRC) and the Australian Health Ethics
Committee (AHEC) of the National Health and Medical Research Council (NHMRC)
are jointly conducting an an inquiry into the uses of human genetic information.
The inquiry will consider whether a regulatory framework is required to:
- protect the privacy of human genetic samples and information;
- provide protection from inappropriate discriminatory use of human genetic
information; and
- reflect the balance of ethical considerations relevant to the collection
and uses of human genetic samples and information in Australia.
The inquiry is due to report its findings to the Government by July 2002.
For further information, contact the ALRC on (02) 9284 6333.
CLONING TECHNOLOGY ACQUIRED
Clone Australia, a company established by two scientists from the University
of Melbourne, has secured a non-exclusive licence to use the nuclear transfer
cloning technology owned by US firm Geron Corporation that was used to create
Dolly the Sheep.
The technology, which Geron purchased from the Rosslyn Institute in Edinburgh,
will be used to clone Australian high-performance cattle and sheep for export
as breeding stock and to produce sheep and cattle breeding stock in China.
Clone Chairman, Dr Malcolm Brandon, said the licence would enable the breeding
of disease-free Australian animals for sale in countries whose stock was infected
with mad cow disease, foot-and-mouth disease and scrapie. He said several animals
had been impregnated with genetic material from top breeding lines as soon as
the licence was signed, and cloned animals are expected to be ready for sale
by the end of the year.
NEW OBESITY AND DIABETES
GENES DISCOVERED
Autogen Ltd has discovered five new genes associated with obesity and diabetes.
Patent applications have been filed for the new genes, which may provide novel
targets for developing treatments for diabetes and obesity.
The new genes, discovered by researchers at Deakin University, are in addition
to the ‘Beacon’ gene for obesity and the ‘Tanis’ gene for diabetes, and bring
Autogen’s portfolio of new gene discoveries in obesity and diabetes to 15.
Professor Greg Collier, Autogen’s Chief Scientific Officer, said that the gene
discovery facility and Autogen’s animal model and exclusive access to human/DNA
samples were enabling the company rapidly to discover new genes that could lead
to new treatments for common metabolic diseases.
CHIF/ABA 2001
The Australian Biotechnology Association and the Commercialising Health Innovations
Forum have formed a partnership to jointly hold the 2001 conference.
Modelled on the American BIO conferences, CHIF brings together venture capitalists
with Australian and Asia Pacific health biotechnology innovators.
Up to 600 delegates are expected to attend the forum to be held in Sydney 15-17
August. For further details, contact Marg Scarlett (03) 9521 8881 or email:
conforg@ozemail.com.au.
GRADIPORE ENDOWS US CHAIR
Sydney-based biotechnology firm, Gradipore Ltd, has announced it will contribute
$500,000 towards a Gradipore Chair in Separation Science at the Texas A&M University,
College Station, USA. Professor Gyula Vigh will be designated the inaugural
chair holder, and the Gradipore endowment will be matched by the university.
PATENT FOR ARTIFICIAL HEART
MicroMedical Ltd has been granted an Australian patent for its artificial heart
technology.
The VentrAssist artificial heart uses a new method of pumping blood which does
not require blood shafts, seals and conventional bearings, effectively eliminating
blood damage and device wear.
The artificial heart device has successfully undergone a series of animal trials
and is expected to be ready for human trials later this year. Patents for the
device are expected to be granted in the USA and Europe in the near future.
A FEAST FOR THE MIND
The first physical event under the FEAST banner is to be held in Canberra on
30-31 May with the theme Enhancing Research through Collaboration and Linkages.
220 Delegates will meet over two days to discuss some of the critical issues
impacting on European-Australian research cooperation.
For further information on this and other forums, see http://www.franc.net.au/feast/activity/
BIOTECHNOLOGY TEACHING TO BE BENCHMARKED
A project to benchmark internationally the current status and teaching of biotechnology
in Australian universities was launched in April with a grant from the Australian
Universities Teaching Committee. The joint study is to be undertaken by Professor
Peter Gray, (University of New South Wales), Associate Professor Ross Barnard
(University of Queensland), and Associate Professor Chris Franco (Flinders University).
“One of the key questions will be to determine the extent to which Australian
universities are offering programs which will meet the needs of the developing
biotechnology industry,” said Professor Gray. “The study will involve a group
who collectively cover the medical, food and agriculture, industrial and environmental
areas of biotechnology. Representatives of a number of biotechnology companies,
as well as several start-up companies, will participate in the study. The effort
will address companies in Australia as well as a set of exemplary companies
overseas.
“The study will focus on industry perspectives on the knowledge, skills and
abilities that they need in new employees and on the industry impression of
their own impact on university courses,” Professor Gray said.
For further information, contact Professor Peter Gray, Tel: (02) 9385 2061,
Associate Professor Ross Barnard, Tel: (07) 3365 4612, Associate Professor Chris
Franco, Tel: (08) 8204 5764 or Ms Anne Howard, Mob: 0403 583 601.
RESEARCH INSTITUTES
SET UP COMMERCIAL ARM
Five Victorian medical research institutes have jointly established a new biotechnology
commercialisation venture, Biocomm International Pty Ltd. The venture has been
granted funding of $4 million over four years by the Victorian Government through
its Technology Commercialisation Program.
Biocomm will invest in and oversee commercialisation of medical research by
its founding members - the Monash University Faculty of Medicine, the Victorian
College of Pharmacy, the Baker Institute of Medical Research, RMIT Life Sciences
and the Peter MacCallum Cancer Institute. The University of Melbourne is also
considering subscribing to the venture.
Biocomm is to be headed by Dr Andrew Gearing of British Biotechnology Ltd,
and its board will include Dr Arthur Emmett, formerly of Ciba Geigy and Stan
Yakatan, a US scientist and entrepreneur.
EDIBLE VACCINE PROGRESS
A Monash University research team has grown a genetically-engineered plant
containing a measles vaccine using a technique that could lead to edible vaccines
for range of viral diseases, including HIV and malaria.
The research, led by Professor Steve Wesselingh and recently published in the
journal, Vaccine, demonstrates that oral vaccination using transgenic
plants is a viable way of developing a novel measles vaccine.
A tobacco leaf was produced which contained a viral protein found in the measles
virus, and when it was processed and fed to mice, their immune systems responded
by producing protective antibodies. Testing has now begun on primates. The protein
is also being developed in a range of foods including rice and lettuce.
Experimental work has also begun on a combination of edible and injectable
vaccines in early HIV and malaria vaccines.
CALL FOR GENE TECH ADVISORY
COMMITTEES
The Interim Office of the Gene Technology Regulator (IOGTR) has called for
nominations for membership of three new committees which are to be established
under the new Gene Technology Act which comes into effect on June 21.
The Gene Technology Technical Advisory Committee (GTTAC) will provide scientific
and technical advice to the GTR and the Ministerial Council and will replace
the current Genetic Manipulation Advisory Committee (GMAC).
The Gene Technology Community Consultative Committee (GTCCC), introduced as
a result of strong views expressed during the consultation process for the new
legislation, will advise the GTR and Ministerial Council on matters of general
concern to the community in relation to GMOs.
The Gene Technology Ethics Committee (GTEC) - will provide advice to the GTR
and Ministerial Council on ethical issues relating to gene technology and any
codes of practice or policy principles developed by the GTR or the Ministerial
Council. http://www.health.gov
Copyright 2001 - AusBiotech
| 