|
Australasian Biotechnology (backfiles)
AusBiotech
ISSN: 1036-7128
Vol. 11, Num. 2, 2001, pp. 26-28
|
Untitled Document
Australasian Biotechnology, Vol. 11 No. 2, 2001, pp. 26-28
BIOPROCESSING
MICROBIAL BIOTRANSFORMATIONS: STEREOSELECTIVE SYNTHESIS OF PHARMACEUTICAL
DRUG PRECURSORS
Shafiq Ahmad, Kylie Henderson, Geoff Dumsday and Michael Zachariou
Code Number: au01026
ABSTRACT
Biotransformations are becoming increasingly popular in the production of enantio-
and regiopure- intermediates for synthesis of complex organic compounds. Whole
microbial cells or enzymes can be used to carry out such specific chemical reactions
that are otherwise difficult to achieve synthetically. Chemoenzymatic synthesis
is of particular interest in the pharmaceutical industry where some steps can
be carried out enzymatically to obtain chiral synthons for further enzymatic/chemical
processing. In this report, we discuss some recent developments in the field
together with a few examples.
INTRODUCTION
A key area of industrial pharmaceutical research is the search for selective
enzyme inhibitors and receptors. Improved knowledge of molecular interactions
has led to an increased understanding of the importance of chirality to the
efficacy of many products. For instance, in a chiral mixture, only one isomer
may be biologically active whereas the other stereoisomer may be responsible
for side effects. In an attempt to avoid such side effects the pharmaceutical
industry is directing much effort in developing homochiral drugs.
Chiral compounds have at least one stereogenic (asymmetric) carbon atom, which
means the molecule can exist as two different stereoisomers and which may have
different biological activity. A readily available pool of chiral precursors
is necessary and needs to be inexpensive and optically active. Chiral precursors
can be prepared by different routes. One route is to obtain them from naturally
occurring chiral synthons. A second route is to obtain them by racemic resolution
of compounds, which can be either carried out by preferential crystallization
or by kinetic resolution using chemical or microbial means. Finally, the chiral
synthons can also be prepared by asymmetric synthesis using chemical or microbial
systems. The advantages of microbial systems over chemical synthesis are numerous
such as stereoselectivity of enzymes and reactions can be performed at ambient
temperature and atmospheric pressure. Such advantages help minimize problems
such as isomerisation, racemisation, epimerisation and rearrangement that may
occur during chemical processing. Furthermore, microbial biotransformations
reduce the requirement of harmful chemicals whilst introducing the possibility
of reusing microbial cells/enzymes.
This report will highlight some of the latest developments and examples of
stereoselective biotransformation to preparation of chiral precursors for chemical
or biological synthesis of pharmaceuticals. For more detailed information the
reader is referred to a number of recent reviews on the topic of stereoselective
biotransformation (Johnson and Wells 1998; Andreas et al., 1999; Ward
and Singh 2000; Azerad and Buisson 2000).
STEROIDS AS CHIRAL SYNTHONS
Steroids are widely used as anti-inflammatory, diuretic, anabolic, contraceptive
and anticancer agents some of which have stereoselective requirements. Chemical
modification of the steroid ring structure imposes many problems due to steric
hindrance and the complexity of the molecule. An alternative approach that obviates
the need for chemical modification of the pharmaceutically active steroids has
been to use microbial systems (Ahmad et al., 1992) which dates back to
the early 1950s (Murray and Peterson 1952). For example, microbial sterol side-chain
cleavage for production of steroid drug precursors, such as the cyclopentanoper-hydrophenanthrene
ring structure, from sterol raw materials such as cholesterol and stigmasterol,
are well known and documented (Mahato and Banerjee 1985; Ahmad et al.,
1991; 1992; Ahmad and Johri 1993; Naito 2000) (Fig 1.). This process (Fig 1)
has always competed with chemical side-chain cleavage of steroids, however microbial
processes (most of them are patented) are preferred because of their stereoselectivity.
The products of partial or complete side-chain degradation are valuable for
microbial or chemical synthesis of most of the steroid drugs or hormones.
Another important process for development of bioactive steroids is, stereoselective
hydroxylations such as 11α-, 11ß- and 16α-hydroxylation, all of which
are now exclusively achieved by microbial means (Mahato and Garai 1997). Yoshioka
and Asada (1994) patented a process for 14α-hydroxylation of androst-4-ene-3,
17-dione (AD) (Fig. 1). A potent inhibitor of breast cancer cells 6ß, 14α-dihydroxy-androst-4-ene-3,
17-dione was achieved by microbial transformation of AD by the genus Myrothecium
(Yoshioka et al., 1994). A novel steroid drug which has demonstrated
strong antitumor (ovarian) activity 14α-hydroxyandrost-4-ene-3, 6, 17-trione
was also produced by oxidation of 6ß, 14α-Dihydroxyandrost-4-ene-3, 17-dione
(Yoshihama 1993) (Fig. 1).
The regio- and stereoselective hydroxylation of progesterone catalyzed by
Rhizopus nigricans is a key step in the manufacture of steroid drugs with
a market value of more then one billion dollars (Mahato and Garai 1997) (Fig.
1). Another approach used in the hydroxylation of progesterone may be the use
of 16α-progesteronehydroxylase from Streptomyces roseochromogenes NCIB
10984 (Barrie et al., 1999). The enzyme could be tested on other steroid
ring structures as well.
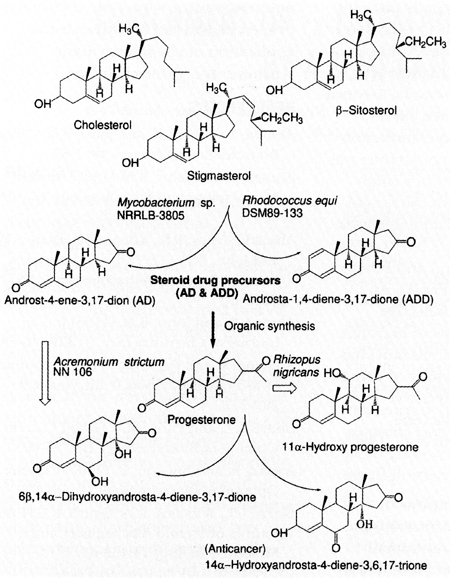
Figure 1. Microbial sterol side-chain degradation and stereoselective
oxidation.
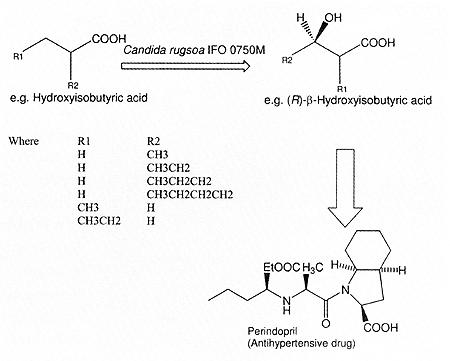
Figure 2. Enantioselective hydroxylation of aliphatic caboxylic
acids.
ALIPHATIC CARBOXYLIC ACIDS AS CHIRAL SYNTHONS
An example of microbial biotransformation of aliphatic carboxylic acids to
produce a chiral synthon is the regio- and enantioselective ß-hydroxylation
performed by Candida rugosa and C. parapsitosis. (R)-ß-Hydroxyisobutyric
acid produced using this reaction is chiral synthon in the chemical synthesis
of antihypertensive drugs. All angiotensin converting enzyme (ACE) inhibitors
(antihypertensive drugs) are synthetic and chiral molecules and without exception
are marketed as a single isomer. For instance, perindopril has five asymmetric
centres and is marketed as one of 32 (25) possible isomers (Fig. 2).
CYCLOHEXADIENE-CIS-DIOLS AS CHIRAL SYNTHONS
The capacity to functionalize cis-diols chemically at every position
in a stereocontrolled manner has served to produce enantiopure precursors, such
as cyclohexadiene-cis-diols, of pharmaceutically important organic compounds
(Fig. 3) (Hudlicky and Thorpe (1996). Stereoselective synthesis of pancratistatin
(antitumor agent) using cyclohexadiene-cis-diol as a precursor has been
described (Hudlicky et al., 1996).
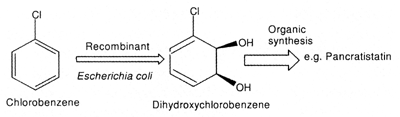
Figure 3. Dioxygenase mediated cis-hydroxylation.
Gibson et al. (1968) reported dioxygenase mediated cis-hydroxylation
of aromatic compounds to cyclohexadiene-cis-diols by Pseudomonas putida.
The dioxygenase gene was cloned in to Escherichia coli to stop further
metabolization of cis-diols and to over produce the enzyme. (Fig. 3).
Recently, an interesting strain of Burkhoderia sp. was reported to utilize
a wide range of carbon sources including toluene, cresol and a range of alkanes
ranging in length from C8 to C25 (Ma and Herson 2000). The catechol 2,3-dioxygenase
gene (catB) was also cloned and sequenced from this interesting strain
(Ma and Herson 2000). This enzyme may have a different substrate specificity
in a different reaction environment.
GENERATION OF CHIRAL SYNTHONS FROM RACEMIC SOLUTIONS USING BIOTRANSFORMATION
One of the major routes of chiral precursors for pharmaceutical drug synthesis
is microbial or enzymatic resolution of racemic mixtures derived either from
natural or chemical sources. A commercial mixture of nitropentanol isomers was
separated using Hansenula subpelliculosa (ATCC 16766) (Morgan et al.,
1999). Nitropentanol isomers are used in chemical synthesis of azole antifungal
compounds. Isopropyl amine and 1,2-isopropylideneglycerol isomers were produced
using Pseudomonas sp. and P. putida respectively (Fig.4A). Isopropylideneglycerol
particularly R isomer is an industrially valuable precursor for a number of
pharmaceutically active drugs, such as ß-blockers, antiviral agents and thromboxane
synthase inhibitor. Isopropylamine is converted to an optically active L-alaninol,
which is a precursor to an important antibacterial agent (Fig. 4B).
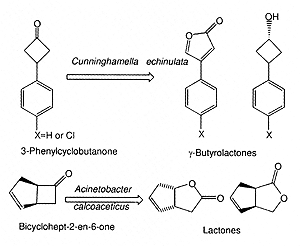
Figure 4. Enantioselective microbial oxidation.
A chemically difficult reaction to carry out is the Baeyer-Villinger (BV) oxidation
of linear or cyclic ketones. The oxidation of 3-phenylcyclobutanone to γ-butyrolactone
was achieved in a single step with the fungal strain Cunninghamella echinulata
NRRL 3655 (Alphand and Furstoss 2000) (Fig. 5). An enantioselective synthesis
of baclofen (muscle relaxant) has been described using optically active γ-butyrolactone
(Mazzini et al., 1997).
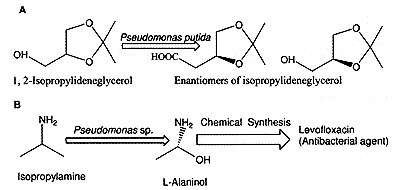
Figure 5. Regioselective oxidation of ketones and resolution.
CONCLUSION
A number of new applications of stereoselective biotransformations are appearing
in the literature. A small sample of these have been presented in this report
to represent a form of bioprocessing used for many decades. This type of bioprocessing
is finding an increasing use in the pharmaceutical industry as it strives for
lower levels of toxicity in its products and, also, in the chemical industry
as it begins to redefine itself as a more environmentally friendly and efficient
sector. The ability of the ubiquitous and versatile microbe to rapidly adapt
to changing environments has now made it a target as a synthetic tool for production
of novel building blocks.
Microbial resolution of racemic mixtures of chemical precursors or final products
has already been established. Microbial transformation or biocatalysis has proven
to be a valuable tool in production of pharmaceutical intermediates, in particular
enantiomerically pure compounds. In some of the cases, solubility of the reactants
and or products in the reaction mixture employing enzymes or microbial cells
remains a critical problem to be solved. Furthermore, stability of microbial
enzyme(s) could be a problem in the chemical environment suited for chemical
reaction.
Developments in microbial biotransformation technology, including new and more
stable enzymes, improvement of existing enzyme systems using site-directed mutagenesis
and search for novel microorganisms with desired enzymatic properties will further
advance the application of chemoenzymatic synthesis in organic chemistry.
REFERENCES
- Ahmad, S., Garg, S.K. and Johri, B.N. (1992) Biotechnology Advances 10:
1-67.
- Ahmad, S. and Johri, B.N. (1992) Applied Microbiology and Biotechnology
37: 468-469.
- Ahmad, S., Roy, P.K., Khan, A.W., Basu, S.K. and Johri, B.N. (1991) World
Journal of Microbiology and Biotechnology 7: 557-561.
- Ahmad, S. and Johri, B.N. (1993) Indian Journal of Chemistry (Sec. B) 32B:
76-69.
- Alphand, V. and Furstoss, R. (2000) Journal of Molecular Catalysis B: Enzymatic
9: 209-217.
- Andreas, L. and Filho, M.V. (1999) Current Opinion in Biotechnology 10:
595-603.
- Azerad, R. and Buisson, D. (2000) Current Opinion in Biotechnology 11:
565-571.
- Berrie, J.R., Williams, R.A., Smith, K.E.(1999) Journal of Steroid Biochemistry
and Molecular Biology 15:153-65.
- Hudlicky, T. and Thorpe, A.J. (1996) Chemical Communication 1993-2000.
- Hudlicky, T., Tian, X., Konigsberger, K., Maurya, R., Rouden, J. and Fan
B. (1996) Journal of American Chemical Society 118: 10752-10765.
- Johnson, C.R. and Wells, G.W. (1998) Current Opinion in Chemical Biology
2: 70-76.
- Kulla, H.G. (1991) Chimia 45: 81-85.
- Ma, Y. and Herson, D.S. (2000) Journal of Industrial Microbiology and Biotechnology
25: 127-131.
- Mahato, S.B. and Banerjee, S. (1985) Phytochemistry 23: 1403-1421.
- Mahato, S.B. and Garai, S. (1997) Steroids 62: 332-345.
- Mazzini, C., lebreton, J., Alphand, V. and Furstoss, R. (1997) Tetrahedron
Letters 38: 1195-1196.
- Morgan, B., Sarikonda, B.R., Dodds, D.R., Homann, M.J. and Vail, R. (1999)
Tetrahedron: Asymmetry 10: 3681-3690.
- Murray, H.C. and Peterson, D.H. (1952) US Patent 2602769 (Upjohn Co. Kalamazoo,
Michigan, USA.
- Naito, A. (2000) Yakugaku Zasshi. 120: 839-48.
- Schulze, B. and Wubbolts, M.G. (1999) Current Opinion in Biotechnology 10:
609-615.
- Ward, O.P. and Singh, A. (2000) Current Opinion in Biotechnology 11:
520-526.
- Yoshihama, M. (1993) Yukijirushi Nyugyo Kenkyusho Hokoku 99: 1-70.
- Yoshioka, H. and Asada, S. (1994) Japanese Patent 06, 153, 987 (Nippon Kayaku,
KK).
- Yoshioka, H., Asada, S. and Fujita, S. (1994) European Patent 599, 658 ((Nippon
Kayaku, KK).
Copyright 2001 - AusBiotech
|
