 |
| About Bioline | All Journals | Testimonials | Membership | News |
|
||||||
|
||||||
Journal of Applied Sciences & Environmental Management, Vol. 10, No. 3, September, 2006, pp. 151-155 The Graft Copolymerisation of Acrylamide onto Cellulose using Enhanced Fe2+ / H2O2 redox initiator system 1Jideonwo, A; *2Adimula, H A 1
Department of Chemistry, University
of Benin, Benin
City, Nigeria Code Number: ja06068 ABSTRACT: Graft copolymers of acrylamide onto holocellulose derived from cocoa (Theobroma cacao) wood meal have been produced using hydroquinone enhanced Fe2+/H2O2 redox system. The addition of hydroquinone to the redox system affected the effectiveness of the redox system to initiate graft copolymer formation. The effects of time, monomer concentration, initiator concentration, and temperature were studied to determine the optimum condition for graft copolymer formation. @JASEM Cellulose is frequently modified in the preparation of a wide range of new materials that have proved to be very useful in several and diverse fields of application (Onishi et al, 2004; Heinrich and Mischnick, 1999; Kato et al, 1999). The presence of three reactive hydroxyl groups on each glucan unit of cellulose makes it relatively easy to modify. One method of modifying cellulose that has been studied extensively is graft copolymerization (Ouajai, et al., 2004). Cellulose graft copolymers are very attractive because their products can readily be made to posses any number of the required properties (Hodano et al, 2003; Okieimen, 2003). Great numbers of grafting methods have been developed, but the free radical methods of generating radicals on the cellulose backbone before grafting have received the greatest attention (Fernandez et al., 1990). The use of ceric ions to generate free radicals which initiate grafting reactions has been one of the most reported (Gupta and Khandekar, 2002). The limitations of this graft copolymerization of acrylamide on cellulose using hydroquinone enhanced Fe2+/H2O2, initiating system. Materials and Methods Ferrous ammonium sulphate, 30% H2O2 and hydroquinone were reagent grade BDH, England, chemicals. Acrylamide was obtained from Burgoyne Urbidges, India and was used without extraction of the polymerization inhibitor. The water used in the reactions was re-distilled. Preparation of Holocellulose: In a typical experiment cocoa wood waste was obtained from farms in Idanre, Ondo State, Nigeria. The dried wood chunks were ground and sieved and material retained on a 60-mesh screen collected. The material was de-waxed by Soxhlet extraction for 24 hrs using a 1:1 v/v mixture of benzene and ethanol. The extractive free wood was washed thoroughly with distilled water and air dried. 5g portion of the extractive free wood was initiating system, the relatively large amount of homopolymers formed, have been reported (Okieimen and Idehen, 1989). Redox initiating systems which reduce the formation of homopolymers have also been developed. Fe2+ /H2O2 is one of the best and most effective redox systems (Mateva and Nikolov, 1991). Reports exist which show that the efficiency of grafting parameters could be increased by the addition of a suitable reducing agent such as hydroquinone into redox initiating systems. Mateva and Nikolov reported in their work the use of hydrazine hydrate to increase the efficiency of the redox system. Although several workers have reported on the graft copolymerization of vinyl monomers on cellulosic material, no information has been found however on the graft copolymerisation to cellulose obtained from cocoa wood waste. Because of the large availability of this material as agricultural wastes, research was initiated on the possible production of simple value added products from it. This work reports on teh heated with 160ml H2O containing 0.5ml acetic acid and 1.5g NaClO2 for 4 hours at 70-80°C. 0.5ml acetic acid and 1.5g NaClO2 were added each hour until a total of 2ml acetic acid and 6.0g NaClO2 have been added. The holocellulose obtained was filtered, washed thoroughly and dried. Graft Copolymerization: Graft copolymerization of acrylamide onto the holocellulose was achieved by an adaptation of the methods described by Mateva and Nikolov (1991), and, Vazquez et al., (1989), for the radical graft copolymerization of vinyl monomers onto polysaccharide using the Fe2+/H2O2 redox system. In a typical reaction, 1 gram of the holocellulose was dispersed in a 100ml 1% w/v aqueous ferrous ammonium sulphate solution for 1 hour at 30oC. The excess Fe2+ salt was drained off by filtration and the holocellulose rinsed with distilled water. The holocellulose on to which Fe2+ was adsorbed was dispersed in 100ml of acrylamide solution of pre-determined concentration for another one hour at the reaction temperature to allow for adequate interaction of the holocellulose fiber with the monomer. 0.5g of hydroquinone and the required amount of H2O2 were then added. The grafting reaction was allowed to proceed with stirring for the pre-determined duration. The graft copolymer obtained was washed several times with distilled water. Extraction of the homopolymer was done in a Soxhlet extractor with ethanol until constant weight was obtained. The graft copolymer was washed with distilled water again and dried first in air and later at 500C in an oven to a constant weight. Determination of grafting parameters: The grafting parameters were determined by weighing. The effect of changes in the reaction condition: initiating system, time, monomer concentration, H2O2 concentration, and temperature on the grafting parameters: grafting efficiency, grafting yield, total monomer conversion into polymers and graft conversion were calculated. Grafting parameters:Apparent grafting yield, G is the weight ratio of grafted polymer to original cellulose:
Grafting efficiency, GE, is the fraction of the total synthetic polymer that is grafted to cellulose:
Total conversion of monomer to polymer, Ct, is the monomer fraction that polymerizes:
Graft conversion, Cg, is the monomer fraction that affords grafted polymer: Wg = weight of graft copolymer sample after extracting the acrylamide homopolymer; Wc = weight original cellulose; Wm = weight of acrylamide monomer; Wcp = weight of crude copolymer product before extracting the homopolymer
The effect of the addition of the reducing agent, hydroquinone, into the initiator system on grafting parameters is presented below: Table 1:The effect of hydroquinone on the efficiency of H2O2/Fe2+ redox system. [H2O2]M = 5 x 10–2; polymerization temperature 60oC; reaction time 2hr.
Results show that the addition of hydroquinone to the Fe2+/H2O2 redox system increased all the grafting parameters studied: %G, %GE, %Ct and the %Cg. The results agree with reports by other workers which showed similar increases in graft yields on the addition of a reducing agent to the redox system (Mateva and Nikolov, 1991). It is considered that the addition of hydroquinone acts in two ways: (I) the reduction of the inactive Fe3+ to the active Fe2+ state as follows:
It is thought that some of the Fe2+ adsorbed onto the cellulose would have been oxidized to Fe3+ by the atmospheric oxygen. This to be expected since the reaction was not carried out in an oxygen-free environment. The addition of a reducing agent, hydroquinone, thus regenerates the Fe2+ / H2O2 system causing the initiation and grafting to continue or (ii) the hydroquinone may also decompose the H2O2 thus: 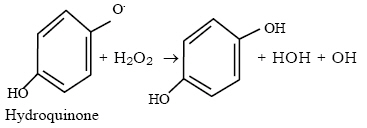
The radicals generated could be thought to be capable of initiating graft copolymerisation by the production of radicals on the cellulose as well as on the monomer. In both cases the graft yield would improve essentially because the hydroquinone has been added into the initiator system. Concentration of acrylamide used 0.2M; polymerization temperature 600C The effect of reaction time on percent graft yield and percent graft efficiency is presented in Fig. 1. An increase in graft yield is observed initially as the reaction time increases from 30 to 60 minutes and less rapidly to 120 minutes when a leveling off is observed. The optimum reaction time for grafting is thus 120 minutes and a longer reaction time will not produce higher graft yield. The amount of polymerization increases with time and this account for the initial rise in graft yields. But the rate would be expected to slow as the acrylamide is used up and the initial radicals generated on the holocellulose backbone are depleted by grafting. The grafting efficiency was actually observed to fall after 120 minutes indicating that more homopolymers are formed in preference to copolymers after 120 minutes. Stopping the reaction at 120 minutes will thus prevent significant quantity of homopolymer formation. Polymerization temperature 60oC; reaction time 2hours Fig. 2 shows the effect of monomer concentration on the graft yield and graft efficiency. The graft yield increased as the monomer concentration is increased up to a maximum monomer concentration of 0.5M. At a higher monomer concentration, 0.6M, the graft yield was observed to fall. The initial rise in graft yield with monomer concentration is expected as there is a higher amount of the acrylamide available for copolymerization. But above 0.5M the excess monomer concentration did not result in graft copolymerization product. This is considered to be due to the fact that under a given initiator concentration the available number of active sites for grafting is constant and the acrylamide molecules compete for the limited sites for grafting, thus at high monomer concentration there is an excess of the monomer molecules relative to the available sites for graft formation hence more of the monomer molecules participate in homopolymerisation. Fig. 2 also shows that the graft efficiency (GE) decreased with increasing monomer concentration. This may be due to an increasing formation of the homopolymer as the concentration of the monomer is increased. Even though the reaction conditions were chosen to maximize the formation of the copolymer, the formation of homopolymers may not have been prevented. The dependency of graft conversion (Cg) on concentration is presented in Fig. 3 as plot of %Graft conversion against monomer concentration. The result shows that the fraction of the monomer that resulted in graft copolymerisation is highest at low concentrations. At this relatively low concentration the few monomer molecules available are readily converted to copolymers. As the acrylamide concentration increased, however, more of acrylamide forms homopolymers resulting in less conversion to the graft copolymer. This confirms the earlier observation that higher concentration of the monomer results in more molecules of the monomer being available to form the homopolymers. The dependency of Cg and Ct on time is shown in Fig. 4. Results showed that the amount of monomer that polymerized (Ct) increased with time. Result showed an initial rapid increase up to 60 minutes and then less rapidly thereafter for the duration of the experiment, 180 minutes. This is expected since polymerization theoretically should continue until all the monomer molecules have been converted given the presence of initiators, although the rate of polymerization would be expected to slow as the initial concentration of initiators are used up. Fig. 4 also showed that the fraction of monomers converted to graft copolymers (Cg) increased rapidly up to 60 minutes and then less rapidly up to 120 minutes when it leveled off. With more grafts formed there is increasingly less amount of active sites for grafting on the cellulose backbone and it would be expected that fewer grafts should result with time. The effect of temperature on graft yield and grafting efficiency is presented in Figure 5. Graft yield (G) increased with rise in temperature probably due to increased decomposition of H2O2, greater mobility of monomer molecules and enhancement of rates of the reactions. The formation of graft copolymers at low temperatures is probably due to increased decomposition of H2O2 by the addition of hydroquinone to the redox system, and the abstraction of H from hydroquinone to generate a radical capable of initiating grafting. The grafting efficiency (GE) was observed to decrease after 600C. This was probably due to termination reactions caused by the increased number of radicals generated in the reaction medium as temperature is raised thus the number of active sites for grafting on the cellulose is decreased. The result of the variation of [H2O2] on the graft yields and graft efficiency is presented in figure 6. The %G and %GE decreased progressively with rising [H2O2] until it levels off at 30 x 10–2 M H2O2. At high [H2O2] there are more radicals produced which may combine to promote termination of radical on the cellulose backbone and the termination of the polymerization reactions. A mechanism for the graft copolymerization of a vinyl monomer, onto a polysaccharide, initiated by Fe (II) / H2O2 has been proposed (Vazquez et al (1989). The results obtained in this study confirm that a high [H2O2] will not increase the graft yields even though there may be an increase in total polymers produced. Conclusion: Results from this work showed that holocellulose derived from cocoa (Theobroma cacao) wood meal are a good substrate for copolymerisation. Cellulose graft copolymers have proved useful in several fields thus value added products could fairly easily be obtained from agricultural wastes that otherwise pose environmental challenge. The addition of hydroquinone to the redox system has resulted in significant improvements in the grafting parameters. REFERENCES
Copyright 2006 - Journal of Applied Sciences & Environmental Management The following images related to this document are available:Photo images[ja06068f3.jpg] [ja06068f2.jpg] [ja06068f6.jpg] [ja06068f1.jpg] [ja06068f5.jpg] [ja06068f4.jpg] |
| |||||||||