
|
Indian Journal of Medical Sciences
Medknow Publications on behalf of Indian Journal of Medical Sciences Trust
ISSN: 0019-5359 EISSN: 1998-3654
Vol. 57, Num. 7, 2003, pp. 311-318
|
Indian Journal of Medical Sciences, Volume 57, Number 7, July 2003, pp.
311-318
Xenotransplantation Ethics and Immunological Hurdles!
U Shankarkumar
HLA department, Institute of Immunohaematology,
13th Floor, K.E.M. Hospital, Parel, Mumbai 400012. India. E-mail:
shankarkumar16@hotmail.com
Accepted on 6-3-2003.
Code Number: ms03006
Exiting new technologies, such as cellular transplantation, organogenesis
and Xenotransplantation are thought to be promising approaches for the treatment
of human disease.1 Remarkable results
have been achieves in the field of organ transplantation over the past 40
years, perhaps inconceivable in the pioneering
days of the 1950's. Factors, which have
contributed to these results, include better
immuno-suppression, matching for HLA, better preservation, and resolution of
most of the technical problems associated with organ transplantation. Scientists
and
transplant surgeons are considering the use of
animals as a source of organs and tissues for transplantation into humans.
This procedure is known as Xenotransplantation. This is
not a new idea. In 1682 doctors have repaired the damaged skull of an injured
Russian nobleman using bone from the skull of a
dog. In 1905 a surgeon from France transplanted slices of rabbit kidney into
a 16-yr. old boy suffering from end stage kidney failures, unfortunately patient
died two weeks
later.1
The growing demand of organs for
transplantation has made scientists to think for an alternative to
allotransplantation especially in heart failures. It has
been estimated that approximately 45,000 Americans under the age of 65 could
be benefited each year from heart transplantation, yet only 2,000 human
hearts are available annually.2 Currently
3,500 Canadians are waiting for donor
organ.1 Hardy attempted the first
cardiac xenotransplant in humans in 1964 with chimpanzee as a donor. Bailey in
1984 did the first human neonatal cardiac xenotransplantion with mismatched
baboon heart that functioned for 20 days. The case is well known a "Baby
Fae".2 Recent advances in our understanding of organ rejection
and in animal genetic modification and cloning made it possible for scientists
and doctors
to consider non-human organs as a viable source of organs for transplant into
humans.1
Early Xenografts failed because the animal organs used were too different
from the recipients tissues. Now the pigs because of its physiological similarities
with humans,
the relative simplicity with which it can be bred and the large litter with ability
to
reproduce faster4 is considered for
Xenotransplantation.3 The size of organ, the blood vessels and
its plumbing is similar to that of humans. This
facilitates easy reconnection of organ during
surgery.4 The cloning of a genetically
modified piglet brings scientists closer to their goal
of xenotranplantation. However the substantial risks and ethical issues
involved need to be considered in the light of its
clinical application as a treatment for end stage
organ diseases.
OVERVIEW
Xenotransplantation would offer an unlimited supply of organs and avert infections
or disease that would occur in human transplant. It will also provide opportunity to
gain expression of extrinsic genes already present or deliberately introduced. However
one potential problem in its clinical application
is dysfunction of transplanted tissue and physiological
limitations.5
Though limited data is available, the porcine hearts can provide physiologic support
for days to weeks in non-human primates. The significant challenge exists in minimizing
the immunological response to the organ transplant.
A: IMMUNOLOGICAL BARRIERS
Two major immunological barriers have impeded the survival of porcine organs
in transplanted in to primates.6
Hyperacute rejection: Which is a consequence of the recipient's
preformed
antibodies binding specifically to the carbohydrate structure of porcine
endothelial cells.
Acute vascular rejection (AVR): It can
also be termed as Delayed xenograft rejection. It is known to occur several days in animal
in which HRA has been prevented.7
T-cell-mediated rejection: It cannot be precisely defined, as T-cell responses
to xenograft have been difficult in most discordant models because of the
problem non-survival of grafts. However
experiments on murine skin and pancreatic islet
grafts show that T cell mediated xenograft
rejection is often vigorous.
Mechanism of HRA, AVR and T cell mediated
rejection Hyperacute rejection (HRA)
The phenomenon of HRA depends on the binding of natural antibody to the
vascular endothelium, fixation of complement by
that antibody and finally, activation of the endothelial and initiation of
coagulation.8 All human beings have naturally
occurring antibodies XNA (Xenoreactive natural antibodies) in their serum, which
are IgM in nature and react with the carbohydrate xenoantigens (Gal-alpha 1-3-Gal)
expressed on the endothelial cells.9 This lead
to complement activation.The XNA found in human serum shares many
characteristics with naturally occurring blood
group
antibodies and are thought to arise due to the exposure to gastrointestinal
bacteria expressing similar carbohydrate
structure.7 Complement factors bind with the
antibodies resulting in the classical pathway
activation of the complement cascade. The
increased levels of C3a and sC5b-9 found during
this time has diagnostic value.11 As a
result, complement factors C3 and C5 are
activated, ultimately leading to the formation of
C5b-9.10 It is observed that levels of C3a and
sC5b-9 are found increased during this time and is of diagnostic
value.11
Acute Vascular Rejection (AVR)
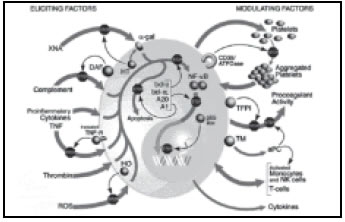
The pathophysiology of delayed xenograft rejection (DXR) is still poorly understood.
The endothelial cell of the graft microvasculature undergoes activation accompanied by
gene up regulation and protein synthesis. The genes that are up regulated include
tissue factor, E-selctin, VCAM-1, ICAM-1 and certain cytokines such as IL-1, IL-6, IL-8
and MCP-1. As a result of increased expression of these proteins, EC undergo
phenotypic changes which make them loose their antithrombotic properties and
attract leucocytes, monocytes stimulate EC and
thus accelerate the ongoing AVR.6
On left are factors that elicit EC and on right are factors that may modulate or play a
role in rejection (Figure 1).12
T-cell Mediated rejection
The crucial molecular interaction required for human T cell action is efficiently
supplied by porcine antigen presenting cells, resulting in an efficient "direct" xenoresponse. Also
the enormous number of pig antigens, recognized as foreign by host
MHC-class-II restricted T cells, provoke a strong
"indirect" xenoresponse. The implication is that, in
vivo, pig xenografts are almost certain to provoke vigorous direct and indirect
T cell xenoresponses with in the first week after
xenotransplantation.7
B: MICROBIOLOGICAL BARRIERS
In March 1997, British researchers reported that pig retroviruses (PERVs)
infected
human kidney cells in vitro and replicated
themselves until the viral particles "were no
longer susceptible to destruction by the human immune
system.12 Retrovirus infection is life long. Two sets of pig retrovirus,
PERV-A and PERV-B are found. They are widely distributed in different pig breeds
and expressed in different tissues, including spleen, kidney and heart, aortic
endothelial
cells, hepatocytes, skin and lung.13 The
pigs may have many unknown retro and herpes viruses in addition to bacteria,
fungi
and parasites capable of xenozoonoses.14
Strategies to overcome immunological and microbiological
barriers:6,7,13
- Prevention of interaction between XNA and epitopes on xenografts
- Interferes with the activation of complement.
- Preserving antithrombotic properties of EC
- Role of platelet inhibitors or antibodies against adhesion molecules.
- Immunosupression.
- Induction of tolerance.
- Haemopoietic chimerism
- Genetic modification of the pigs.
- Screening the pigs for known viral risks.
Scientific developments to overcome barriers
To prevent the XNA interaction between XNA and xenografts following developments
have been achieved. (a) Immunoadsorption through columns containing gala-
alpha
(1-3) gal linkages7 or protein-A
immunoadsorpiton.18 (b) Removal of xenoreactive antibodies via perfusion
of
the recipients, blood through pig
organs.15 (c) Intravenous infusion of carbohydrates, to
saturate XNA binding sites before
transplantation.7 (d) Decreased expression of the
gal alpha (1-3) gal epitope on porcine cells. (e) The expression of the
human fucosyltransferase. in transgenic mice and pigs resulted in a high expression
of the H antigen, together with a strong reduction of
the H antigen, together with a strong reduction in the expression of the Gal
alpha
1,3 Gal structure.15
Inhibition of complement activation is successful by using variety of compounds
like soluble complement agents such as FUT-175, cobra
venom,16 drug like sesquiterpene also called
K7616 and monoclonal antibodies directed against
complement.6 In vitro studies have shown that human RCAs such as
CD55, CD46 and CD59 can prevent complement mediated lysis when expressed on
the surface of transfected xenogeneic cells. Transgenic pigs expressing DAF,
CD59,
or both molecules have been generated by several different
groups.7 Preventing EC activation in graft by inhibition of NF-kB,
a transcription factor has been successfully achieved by transfecting porcine
EC with
anti-apoptotic genes, such as A 20, bcl-2, and bcl-xL-genes. EC have been shown
to be resistant to activation when exposed, in vitro to activating stimuli like
TNF alpha.
Chicken IgY antibodies have shown to block the complement mediated lysis of PAECs
by human serum and inhibit antibody dependent cell mediated lysis of PACEs by
heat inactivated human serum plus peripheral blood
leukocytes.19 Combination of 1-BI and PGI2 results in moderate inhibition
of
platelet aggregation, without changes in
coagulation. Heparin leads to good inhibition
thrombin-induced platelet aggregation at doses
that cause only minor prolongation of
PTT.17 Use of conventional of
conventional
immunosuppression and hDAf transgenic pigs as organ donors have
demonstrated greater survival in
primates.6 Induction of mixed hematopietic chimerism has
been demonstrated to be an effective method of inducing permanent tolerance of
T and B
cells in closely related concordant. Experiments
on mice have shown improved porcine hematopoietic
engraftment.20 Controversies exist about the PERV transmission,
studies involving cellular porcine material- islet
cells, fetal neuronal cells, extracorporeal liver
cells show no evidence of PERV transmission in any of the animals or human
recipients.21
However, an ELISA test for detection of PERV has been developed by immunization
of rabbits with a peptide corresponding to the C-terminal 19 a of the 10 kDa
(p10) nucleocapsid (Nc) portion of the Gag
polyprotein.22 To minimize the spread
of PERV, Prater's team has used a line of pig- miniature swine, which are unable
to
spread PERV.4 Bio Transplant and Novartis AG,
has also bred pigs it says are incapable of
passing viruses to humans.14 Retroviruses
produced in non-primate species frequently carry glycoproteins on their envelope
with carbohydrate moieties that reflect the biosynthesis capacities of the host
organism. Once introduced into the human blood
steam, these virus particles will be opsonised by
the anti-Gal alpha1, 3-Gal antibody, and be inactivated. A similar mechanism
would protect humans against pig endogenous retroviruses that can be mobilized
from
the
pig genome, and be secreted from a transplanted pig organ into the
recipient.15
C: ETHICAL ISSUES
Ethics is broadly defined referred as a system of moral, scruples, principles or values
that in itself defines what is right or good
behavior: thus, it is appropriate on this level to deal
with such philosophical topics as religion,
political, liberty, human rights and animal
rights.24 Ethics and economics are conjoins, as
they are very much interrelated. Economics is the science of human behavior that
generally deals with the production, distribution
and consumption of commodities, and the applicability of such market mechanisms
as the well-known law of supply and demand. Although the animals that may
become sources of organ often are thought of as commodities, human organs generally are
not so regarded.24
Some of the important ethical issues are briefly described below:
Emotion and Euphemism: Raising the animals for the purpose of "viciously"
killing them and "selfishly" taking their organs
raises different issues. Animal right activists
almost ghoulish delight in showing pictures of mistreated laboratory animals.
On the
other hand, those who favor Xenotransplantation loosely use the word "donor" to
refer the animal that supplies the organ. Its is euphemism bordering on mockery,
however to refer to an animal which is killed solely
so
that its heart, for example, may be put into another creature's body- as a "donor".
In genesis, God declares: "Let us make men in our image and likeness to
rule the fish of the sea, the birds of heaven, the cattle, all wild animals
on earth, and all reptiles that
crawl upon the earth". Aristotle argued that
the animals were inferior to people, and that people therefore had a right to
own and use animals as they saw fit. Both Judeo-Christian teaching and Greek
philosophy agreed
that cruelty to animals was wicked in itself, and furthermore was demeaning to
humans. The Islam and Orthodox Judaism are similar and well known with respect
to pigs. It is fair
to say that these views express a social consensus that exists to this
day.24
Xenozonooses and Public Health Risks
posed: It is well known that microbial traffic has emerged on the planet.
The diseases like AIDS and vCJD have emerged in human species, which is traced
back to animal
origin. These potential viruses do not cause any disease pathology in animals
but have been found to endanger the human race. Pigs may have hundreds of unknown
viruses and
by transplanting organs we are welcoming the entry of such viruses, which are
usually kept at bay by natural barriers. Once these
viruses enter the human body are likely to cause newer diseases in transplant
recipients. Also it is possible that the virus on entering
human body may take its route to care takers of recipients and individuals closely
associated. This may lead to epidemics of viral
diseases
of unknown pathology in the society. Measures to institute life long
surveillance programs for organ recipients may
inflict workload on the Public Health machinery
and involve profound cost.12 However
some authors feel that xenotransplantion will
reduce the risk of disease transmission. The
deadly viruses such as HIV, HBV, HCV, EBV, CMV would not be transmitted as the
donor
is animal origin.14
Allocation of organs: Need and not
finances should dictate organ allocation. The
wealth and powerful now get first access to transplants. Therefore some framework
is must for equity in the allocation of organs as per top priorities. Legal concerns include
the patenting. It is likely that some corporations will develop monopolies over the
artificially engineered and created species.24
Consent and Psychological trauma:
Patients who have been told they will surely die
without receiving a transplant are likely to consent
to virtually any treatment at all, which may not be their true consent. On receiving
the xenograft, both pre and post operation counseling is necessary to prevent
the individual from emotional crippling, as they try to accept images of themselves as
"part animals". Equally important is that they
are accepted by society.24
The issues such as compensation to the xenogenic infected recipient; cost
involved in the long-term treatment of such infected
persons, establishment of tissue blood banks for storage of organs cannot be
neglected. The xenotransplants are much more
costly than allotransplantion. The cost for one xenograft in year 1995 was estimated
to be 2,50,000 dollars. Considering these issues, a huge cost burden is likely
to be imposed
of society.12
CONCLUSION
The clinical Xenotransplantation may achieve its targeted goal of extended
graft survival and remain 3 to 5 years from clinical
trails but must persevere under the consideration of and often is spite of scrutiny
by its most demanding critics. Success has a hundred fathers; failure is an
orphan.2 An article mentioned below reflects the
present consensus of human race. Considering all
the possible risks and ethical issues involved people are eager to receive xenografts.
A study was conducted in Sweden with an aim to study the attitude of patients
waiting
for transplantation versus the general public. And the results were as follows:
60% expressed a positive attitude towards receiving an animal kidney graft with
the same degree
of risk as a human kidney graft, compared with 66% for the patients. The proportion
in favor of receiving a heart remained 60% for the public, but rose to 70% for
the patients, If
a human heart was not available, 61% of public were for the use of an animal
heart, compared with 73% in the patient group. A majority
of the respondents would accept a transplant
from an animal, provide the result and risk of infection were the same as with
a human transplant. Xenotransplantation in India is
still at its infancy eventhough in our Hindu Mythology Lord Ganesha, that reveals
a success story of xenotransplant. Considering the number of patients added
and waiting
for solid organ transplantation every year in
India it is mandatory to initiate research in this
field for the betterment of the ailing patient population.
REFERENCES
- Tucker A, Belcher C, Moloo B, Bell J, Mazzulli
T, et al. The production of transgeneic pigs for potential use in clinical
transplantation: baseline clinical pathology and organ size
studies. Xenotranaplantation 2002;3: 203-8.
- Michler RE. Xenotransplantation : Risks,
clinical potential and future prospects. Emerg Infect
Dis 1996;2:64-70.
- Cozzi E, Whitle DJ. The generation of
transgeneic pigs a potential organ donors for humans.
Nat Med 1995;1:964-6.
- Bijal PT. Cloned pigs modified for use in
human transplants. National Geographic today.
2002;3:http://news.nationalgeographic.com/news.html
- Platt JL. Physiologic barriers to xenotransplantation. Trans Proc
2000;32:1547-8.
- Cozzi E, Masroor S, Soin B, Vial C, White
DJ. Progress in Xenotransplantation. Clin Nephrol
2000;53:13-8.
- Dorling A, Riesbeck K, Warrens A, Lechler
R. Clinical xenotransplantation of solid organs. Lancet 1997;349:867-71.
- Auchincloss H. Xenogeneic transplantation.
A review. Transplantation. 1988;46:1-20.
- Sandrin MS, Vaughan HA, Dabkowski PL, Mckenzie IF. Anti-pig IgM antibodies
in
human
serum react predominantly with Gal (alpha 1-3) Gal epitopes. Proc Natl Acad
Sci
USA 1993;90:11391-5.
- Platt JL, Fishel RJ, Matas AJ, Reif SA,
Bolman RM, Bach FH. Immunopathology of hyperacute xenograft rejection in
a swing to primate model. Transplantation 1991;52:214-20.
- Loss M, Vangerow B, Schmidtho J, Kanz
R, Jalahi A, et al. Acute vascular rejection is associated with systemic
complement activation in a pig to primate kidney xenograft
model. Xenotransplantation 2000;7:186-96.
- Alix Fano MA, Cohen MJ, Cramer M, Greek
R, Kaufmann SR. Pigs, Primates and Plagues: A lay persons guide to the problems
with animal to animal organ transplants. Medical Research Council Publications
(MRMC) UK.
http://www.mrmcmed.org/pigs.html
- Stoye J. No clear answers on safety of pigs
as tissue donor source. Lancet 1998;352:666-7.
- Parkins K. Animal to human transplants
a creation of Frankenstein's monster. http://www.heureka.clara.net/gaia/x-trans.html
- Joziasse DH, Oriol R. Xenotransplantation:
the importance of the Gal alpha 1,3 Gal epitope in hyperacute vascular rejection.
Biochem.
Et Biopysica Acta 1999;1455:
403-18.
- Starzl TE, Tzakis A, Fung JJ, Todo S, et
al. Prospects of clinical xenotransplantation. Transplant Proc 1994;26:1082-8.
- Alwayn IP, Appel JZ, Goepfert C, Bahler
L, Cooper DK, Robson SC. Inhibition of platelet aggregation in baboons: therapeutic
implications for xenotransplantation.
Xenotransplantation 2000;7:247-57.
- Ramos A, Reiez JC, de Francisco AL,
Gomez-Fleitas M, Arias M. Removal of xenoreactive antibodies by protien A
immunoadsorption: experience in 22 patients.
Xenotransplantion 2000;7:14-20.
- Fryer J, Firca J, Leventhal J, Blondin B,
Malcom A, et al. IgG antiporcine endothelial cell antibodies effectively
block human antiporcine xenoantibody binding.
Xenotransplantation 1999;6:98-109.
- Yang YG, Chen AM, Garret LJ, Sergio JJ, et
al. Development and analysis of transgeneic mice expressing porcine hematopoietic
cytokines:
a model for achieving durable porcine hematopoietic chimerism across
an extensive xenogeneic barrier. Xenotransplantation 2000;7:58-64.
- Birmingham K. FDA subcommitte finds no evidence of PERV transmission. Nat
Med 1999;5:855.
- Krach U, Fisher N, Czanderna F, Kurth R,
Tonjes RR. Generation and testing of a highly
specific anti-serum directed against porcine
endogenous retrovirus nucleocapsid.
Xenotransplantation 2000;7:221-9.
- Persson OM, Persson NH, Ranstam J,
Hermeren G. Attitudes toward xenotransplantation
patients waiting for transplantation versus the
general public. Transpl Int 2001;14:334-42.
- Kress JM. Xenotransplantation ethics
and economics. Food Drug Law Journal 1998;53:353-84.
- Salomon DR. The US public health
service guidelines for xenotransplantation advances
and limitations. Xenotransplantation 2001;8:86-9.
Copyright 2003 - Indian Journal of Medical Sciences.
|
