 |
| About Bioline | All Journals | Testimonials | Membership | News |
|
||||||
|
||||||
Indian Journal of Pharmacology, Vol. 37, No. 3, May-June, 2005, pp. 148-154 Education Forum L-Glutamic acid and glutamine: Exciting molecules of clinical interest Kulkarni Chanda, Kulkarni KS, Hamsa BR Department of Pharmacology, St. Johns Medical College, Bangalore Date of Submission: 05-Jul-2004 Code Number: ph05038 Abstract Glutamine is one of the most abundant amino acids and participates in a variety of physiological functions, namely - as a major fuel source for enterocytes, as a substrate for neoglucogenesis in kidney, lymphocytes, and monocytes, a nutrient/substrate in muscle protein metabolism in response to infection, inflammation, and muscle trauma. Studies evaluating the role of glutamine have confirmed it's participation in maintaining mucosal integrity of the gastrointestinal tract following it's administration in patients with major bowel surgery. The role of glutamine as a protective agent in hepatobiliary dysfunction and as a supplement in total parenteral nutrition is well established, particularly, in patients under intensive care. L-Glutamic acid (L-GA) physiologically exists as glutamate. Glutamate along with glutamine plays a major role in amino acid metabolism and thus in maintaining nitrogen balance in the body. Glutamate is a well-established excitatory neurotransmitter in the central nervous system. There has been convincing evidence on protective activity of L-GA and α-ketoglutarate in vincristine-induced neurotoxicity. Based on the above information, a large number of studies have been carried out. The findings of recent clinical studies are presented below. Looking at the wide profile of activity, it has been proposed that though L-GA and glutamine were once considered nonessential for health, may now be considered as - 'conditionally essential' amino acids. While complete therapeutic role is yet to be elucidated, it may be anticipated that L-GA and glutamine may prove to be exciting molecules of interest to clinicians. The future research may therefore be directed at confirming the above activities and at investigating their role in other clinical conditions. Keywords: Amino acid; glutamic acid; glutamine Introduction L-Glutamic acid (L-GA), a seaweed ingredient, identified in 1908 by Japanese scientists responsible for enhancing flavor for food is now best known scientifically as monosodium glutamate (MSG). The sodium salt of glutamic acid (GA) is called as MSG.[1] The other names include - S-(+)-GA, L-GA, 2-aminoglutaric acid, and an anionic form of MSG at physiological pH known as glutamate.[2] MSG, a derivative of GA, is reported to be a naturally occurring nutrient in many foods and is increasingly used in food processing and home cooking in the western world.[3] Its commercial use to improve food palatability for humans is well documented.[4] The presence of free form of glutamate, not linked to protein is said to enhance flavor in food.[5] MSG is known to produce a unique taste sensation termed ′UMAMI′ the fifth taste, i.e. savory or brothy taste present in tomatoes and cheese. Free glutamate content is said to increase during the process of natural ripening and bring about a fuller taste in many foods, the basis behind is not known.[1] It is also called as ′palate pleaser.′ Studies on taste have shown that infants to adults enjoy taste of foods containing glutamate, which is said to be 10 times more in breast milk compared to cow′s milk. Glutamine is the most abundant amino acid present in the body. It is also known as levoglutamide, L-GA 5 amide, L-(+)-2-aminoglutamicacid.[6] It is synthesized in the body from GA and ammonia in an energy requiring reaction. Although nonessential in health, glutamine is conditionally essential in stress and illness.[2] Studies over the last several years have explored the physiological role and therapeutic utility of these molecules in various disease conditions. In this review, an attempt is made to briefly discuss the results of these studies and highlight the future potential for therapeutic utility of L-GA and glutamine, which are physiologically and biochemically inter-related. Physicochemical properties GA is a dicarboxylic acid[2] and is grouped under branched chain amino acid with acidic chain.[7] L-GA is an aliphatic amino acid undergoing degradation in the body to glutamine, which is an amino acid with uncharged polar side chains. Glutamate At physiological pH, GA exists in anionic form and is referred to as glutamate. It is found on surfaces of proteins and it plays a central role in transamination reactions and equilibrates with corresponding keto acid. It is odorless and is best classified as food additive by Joint Expert Committee on food additives advisory board to WHO and FAO.[1]
Glutamine Synthesis and metabolism in the human body Glutamate Glutamate is also synthesized in the body through transamination of α-ketoglutarate by accepting amino groups from amino acids, first step in metabolism of amino acid.[7] 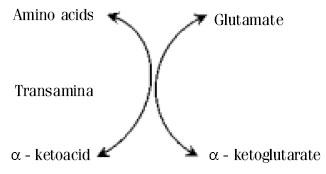
Catabolism
Glutamine Glutamic acid + NH3-------------------
>Glutamine Glutamine is strongly concentrated in skeletal muscle making up to
60% of
free amino acid pool in skeletal muscle.
Natural source Glutamate is found in abundance in both ′free′ and ′bound′ forms in all natural food stuffs - meat, poultry, fish, cheese, milk (including human breast milk), tomatoes, mushrooms, and many other vegetables - peas and broccoli.[1] The bound form of glutamate is linked to other amino acids in proteins present in, muscles, hair, and skin. Glutamate in the human body is considered essential for protein repair, regeneration, and growth. In an average adult, the body content of glutamate is around 1.5-2 kg, mainly in the bound form. The free form of glutamate (i.e. not linked to protein) in food is known to enhance food flavor and its content is said to increase during natural ripening of fruits. It is not a tenderizer or preservative and cannot make inferior food taste good. It has no distinctive smell.[1] Commercial source for production of glutamic acid GA was first produced in Japan in 1908 by hydrolysis of wheat, gluten or Soya bean protein. The fermentation process was invented by Kyowa Hakko Kogyo in 1957, while Ajinomoto Company produced GA synthetically.[11] Large quantities of GA is made by fermenting molasses from sugar beet or sugar cane, a process similar to making wine, beer, sauce, and vinegar.[1] A nonpathogenic species of Coryneform bacterium Corynebacterium glutamicum was originally isolated as an L-glutamate producing bacteria and is now used for industrial fermentative production of various amino acids.[12] Mutations in lts A gene cause a growth defect and induce L-glutamate overproduction by C. glutamicum [Figure - 1]. Other organisms identified to produce L-glutamate are Brevibacterium, Arithrobacter, and Microbacterium.[11] Physiology, pharmacology, and therapeutic role of L-GA and glutamine Central nervous system Glutamate through AMPA and kainate receptors mediate fast excitatory synaptic transmission in the CNS and through NMDA receptors, it mediates slow excitatory response, which plays a role in long-term adaptive and pathological changes in the brain involving synaptic plasticity.[13] Elevated levels of extracellular glutamate can induce seizures and excitotoxic neuronal cell death. In glioma and other brain tumors, excessive glutamate release from tumor cells may be responsible for tumor associated necrosis and also possibly to seizures caused due to peritumoral and tumoral brain tissue.[14] A putative abnormality of glutamate metabolism is thought to be implicated in the pathogenesis of amyotropic lateral sclerosis. It is suggested that accumulation of toxic levels of glutamate, an excitatory neurotransmitter in the synapses may cause neuronal death through calcium-dependent pathways in this condition. However, no benefit has been shown with glutamate antagonists, dextromethorphan, and lamotrigine. Several clinical trials with riluzole, which modulates glutamate transmission, have interestingly shown striking improvement in survival among these patients.[15] Imbalance of glutamatergic excitatory control of motor neurons is reported to be responsible for neuronal damage in motor neuron disease; hence antiglutamate agents such as riluzole and gabapentin have been proposed to have protective effects against this neurodegeneration.[16] Involvement of multiple neurotransmitter systems is also postulated in relation to Alzheimer′s disease, which includes glutamate along with acetylcholine. It is also known that dopamine (DA) interacts with other key systems such as glutamate in Parkinson′s disease. This explains the rationale for pallidotomy diminishing the net excitatory input of glutamate on to the dwindling DA neurons and may require further studies.[17]
Furthermore, Glutamate is accepted as a precursor for the inhibitory neurotransmitter gamma aminobutyric acid (GABA). Differential expression of two genes from hippocampal granule cells, encoding different molecular forms of GA decarboxylase (GAD), GAD65 and GAD67 after kainic acid-induced seizures in rat, has been demonstrated. Such progressive and sustained enhancement of expression of both GAD65 and GAD67 messenger mRNA is suggested to augment GABAergic neurotransmission supporting self-protective, anticonvulsive mechanisms in limbic epilepsy.[18] Metabolism Ammonia metabolism and nitrogen balance Glutamine provides a nontoxic storage and transport form of ammonia. The formation of glutamine occurs primarily in the muscle and liver. At all times, there is net output of glutamine from muscle representing the disposal of amino groups from branched chain amino acids. Glutamine formation is a major mechanism for the removal of ammonia in the brain.[7] Studies have shown that the glutamine supplementation improves nitrogen balance in patients who underwent major surgery or bone marrow transplant.[19],[20] Also a reduced incidence of infection and length of hospital stay have been demonstrated. Further, the lower levels of glutamine in skeletal muscle injuries have been said to indicate its role in the synthesis of muscle protein. Protein metabolism Glutamate and glutamine transporters in skeletal muscle and heart appear to play a role in the control of the steady-state concentration of amino acids in the intracellular space probably through osmotic signaling mechanisms to regulate whole body protein metabolism.[21] The possible role of glutamic acid decarboxylase antibodies (GADA) in type-I diabetes has been suggested in relation to female gender, older age, and the HLA-DR3/DQBFNx0102 haplotype. This conclusion is based on the strong humoral immune response to GAD, which reflects on propensity to general autoimmunity in this condition, rather than excessive beta cell destruction.[22] Reproduction and growth Glutamine and glutamate are shown to play an important role in fetal and placental metabolism.[23] Since milk contains good amount of glutamate in free form, it is proposed to be important in postnatal development.[2] Further, supplementation of glutamine is suggested to offer significant benefits in low birth weight infants including critically ill patients especially in terms of decreased nosocomial infections.[23] Glutathione, a free-radical scavenger synthesized from glutamate in erythrocytes, hepatic tissue, and intestinal mucosa, is found to be depleted in children with low birth weight and kwashiorkor. Therefore, supplementing glutathione and other amino acids have been proposed to be useful in maintaining the integrity of immune system and normal health.[2] Gastrointestinal secretions Glutamine is claimed to participate in the maintenance of gut barrier function modulated through the synthesis of nucleotides, glucosamine, and mucus glycoprotein. The GIT is the principal organ of glutamine utilization with 12-13% of circulating glutamine and 50-60% of enterally supplied glutamine. Glutamine is considered to act as a major fuel and primary source of energy for enterocytes and to enhance mucosal immunologic function. Its presence is shown to stimulate blood flow to the gut, maintain mucosal integrity, prevent villous atrophy, bacterial translocation, and subsequent pro-inflammatory responses. Gut, the main organ for nitrogen processing in the body has been demonstrated to contribute significantly to the supply of glutamine to peripheral tissues. Impaired gut function is said to be associated with significant fall in glutamine levels, resulting in impaired ability to incorporate amino acids into protein. Clinical trials have shown that glutamine enriched solutions improve nitrogen balance and gut morphology.[2],[19],[23] There is reported evidence on the reduction in intestinal mucosal permeability and atrophic changes following administration of TPN containing glutamine dipeptide.[24] Glutamine deficiency is shown to be associated with mucosal atrophy and loss of function. In surgical stress, glutamine uptake by the GIT is known to increase.[2] Immunity In addition to the role of glutamine in immune function in the gut, it is reported to act as a source of nutrition for cells of immune system released from muscles, adipose tissue and other sources. It is said to help in maintenance of general immunocompetence by supporting synthesis of cellular proteins of the immune system and hepatic acute phase protein response. In sepsis, glutamine uptake, and utilization are known to fall with increase in bacterial translocation.[2] L-Glutamine as precursor of GABA has been stated to have antistress activity and to improve cell-mediated immunity in several studies through nucleogenesis of rapidly proliferating cells.[25] It is claimed that such activity may be useful in the treatment of HIV. Respiration In an animal study, it was demonstrated that GA supplementation reduced and brought heart to body weight ratio to normal which was raised in hypoxia and also GA-treated animals showed higher resistance to fall in rectal temperature than control animals when they were subjected to cold stress. These findings suggest that glutamate in optimal doses may enhance tolerance to hypoxia and cold.[26]Anticancer activity The prevalence of cancer-related fatigue in the absence of antineoplastic therapy is said to be 40-75% and is reported to be due to circulating cytokines such as cathepsin-D and Tumor necrosis factor (TNF) (also called asthenins) are proposed to be responsible for metabolic process underlying muscle mass breakdown observed in cancer patients. It is suggested that nitric oxide could reduce the levels of cathepsin by modulating its synthesis and therefore may benefit cancer patients with fatigue. A double blind placebo-controlled cross over study with oral administration of arginine-glutamate 6 g/day for 6 weeks was reported to increase the endogenous production of NO in patients with endothelial dysfunction. However, the relation between the asthenins and improvement in fatigue is said to require further studies.[27] In 2000, Oldham et al. have shown superior anticancer activity of paclitaxel an anticancer agent when combined with L-GA in human breast cancer. It′s anticancer effect has been attributed to it′s ability to produce favorable pharmacokinetic profile and distribution of paclitaxel. The L-GA conjugated with paclitaxel is known to form a new anticancer called poly (L-GA)-paclitaxel (PG-TXL) with superior antitumor activity, favorable pharmacokinetic properties and/or mechanism of action different from that of Paclitaxel alone. It is suggested that superior activity of PG-TXL may be due to continuous release of paclitaxel.[28] In a double blind placebo-controlled study, administration of GA prevented the vincristine-induced neurotoxicity, a well-known principal limiting side effect.[29] The loss of tendo-Achilles reflex as an objective parameter of vincristine-induced neurotoxicity was reported to be significantly higher in placebo group as compared to GA group. Intrα-thecal administration of vincristine produced ascending paralysis and death, which was prevented by i.v. GA. Inhibition of disruption of microtubular structures by GA have been proposed to play a role. Critical illness Glutamine has been reported to be of value in the intensive care of critically ill patients. It is shown to improve nitrogen retention, hepatic protein synthesis and reduced protein degradation coupled with increased glutathione and hence protection of enterocytes through antioxidant action and hence is proposed to subserve as an organ-specific amino acid. It is said that enteral glutamine supplementation preoperatively for 10 days reduces infective complications and length of stay in the hospital.[2] Coagulation GA is present in coagulation factors like II, VII, XI, X as V-carboxyl glutamate residues without which these factors cannot be activated. Hence GA is reported to play an important role in coagulation.[13] Endocrine Significant amounts of GA present in prostate gland are said to be implicated in normal function of this gland. In a study involving 45 men, who were given GA in doses of 780 mg for 2 weeks and 390 mg for the next 2.5 months in combination with equal amounts of alanine and glycine, has shown significant improvement in symptoms associated with hyperplasia of prostate. Hence GA is claimed to benefit patients with prostatic hyperplasia.[30]Adverse reactions Modern day research completely affirms the safety of sodium salt of GA (MSG) for the general population.[1] Reported evidence suggests the following. Dose-related adverse reactions The threshold range for I.V. dose is 25-125 mg for minimum symptoms to occur after 17-20 s. At suprα-threshold dose, tightness, pressure over malar, zygomatic, and retrobulbar areas similar to post-anesthetic numbness, burning sensation over chest with or without spreading to the neck, shoulder, forearms, abdomen, and thighs, have been reported in the absence of ECG abnormalities.[31]
Sensitivity reactions
Excitotoxicity The mechanism proposed to be involved includes - activated receptor allows influx of Ca2+ leading to activation of destructive process. Also this mechanism is said to be responsible for neuronal death in stroke, ischemia, head injury, and hypoglycemia, during which massive release and impaired reuptake of glutamate in the synapse occurs leading to excessive stimulation of glutamate receptors and subsequent neuronal death. Seizure Chinese restaurant syndrome (CRS) Chinese restaurant asthma
Psychological reactions
Hyperglutaminemia and hyperammonemia Miscellaneous Potential uses Taking into account the biochemical, physiological, and pharmacological aspects of L-GA and glutamine, these may be proposed to be useful molecules in several clinical conditions. However, there is no known deficiency of GA and Glutamine encountered in clinical practice. Food industry
Gastrointestinal disorders
Cancer Studies have shown that GA may be beneficial as an adjuvant to anticancer drugs.
Parenteral nutrition In many studies, glutamine has been used as a nutritional agent in parenteral therapy. Since glutamine is unstable, its stable derivatives like glutamine dipeptide or α-ketoglutarate were employed and is found to be effective in:
Neurological conditions In muscular dystrophy, epilepsy, psychiatric disorders, mental retardation, and Parkinsonism the agents, which modulate glutamine transmission are proposed to be useful.[13] Future directions Although GA and glutamine have been thoroughly researched as ingredients of food supplies and as nutrients, their evaluation as therapeutic agents appears to be incomplete. It may thus be concluded that further research is required to explore and examine more closely the potential role of L-GA and glutamine as molecules of clinical utility, particularly, in critically ill patients. References
Copyright 2005 - Indian Journal of Pharmacology The following images related to this document are available:Photo images[ph05038f1.jpg] [ph05038t1.jpg] |
| |||||||||