
|
Indian Journal of Pharmacology
Medknow Publications on behalf of Indian Pharmacological Society
ISSN: 0253-7613 EISSN: 1998-3751
Vol. 37, Num. 4, 2005, pp. 227-231
|
Indian Journal of Pharmacology, Vol. 37, No. 4, July-August, 2005, pp. 227-231
Research Paper
In vitro antioxidant effect of Globularia
alypum L. hydromethanolic extract
Khlifi S., Hachimi Y.E.l., Khalil A., Es-Safi
N., Abbouyi A.E.l.
Laboratoire de Biochimie Appliquée et Biotechnologie. Faculté des Sciences, EL Jadida
Correspondence Address: Laboratoire de Biochimie Appliquée et Biotechnologie.
Faculté des Sciences, EL Jadida, elabbouyi@hotmail.com
Date of Submission: 05-Jan-2005
Date of Decision: 15-Jan-2005
Date of Acceptance: 22-Apr-2005
Code Number: ph05058
ABSTRACT
Objective : To investigate the in vitro antioxidant activity of the hydromethanolic extract of aerial parts (leaves and stems) of Globularia alypum L. toward linoleic acid emulsion and human low-density lipoproteins (LDL) peroxidation.
Materials and Methods : Lipid peroxidation was carried out in the presence of G. alypum hydromethanolic extract (10 and 100 µg of extract/ml). CuSO4 (10 µM) was used as the oxidation initiator. Conjugated dienes (CD) formation and oxygen consumption were assessed for monitoring the antioxidant properties of the plant extract. Butylated hydroxytoluene at 50 µg/ml was used as standard antioxidant. Quantification of total polyphenolic compounds was carried out according to the Folin-Ciocalteu method.
Results : The hydromethanolic extract of G. alypum exhibited significant antioxidant effect. There was a significant inhibition of CD formation in copper ions-mediated linoleic acid emulsion as well as human LDL peroxidation. Analysis of the plant extract revealed a high amount of polyphenols, suggesting a possible role of these compounds in the antioxidant properties.
Conclusion : The obtained results suggested that G. alypum could be a potential source of antioxidants. Further investigations are in progress to determine the active constituent(s).
Keywords: Conjugated dienes, linoleic acid, lipid peroxidation
INTRODUCTION It is well known that reactive oxygen species (ROS) are involved in many pathological disorders such as atherosclerosis and related cardiovascular diseases,[1] diabetes,[2] and cancer.[3] Reactive oxygen species, generated in vivo mainly by neutrophils, macrophages, and xanthine-oxidase system, appear to be responsible in these illnesses by inducing lipid peroxidation via a chain reaction process.
Most living species have protective systems against oxidative stress and toxic effects of ROS. Several studies have demonstrated that the antioxidant properties of plant compounds could be correlated with oxidative stress defense.[4],[5] Thus, antioxidant compounds can be used to counteract oxidative damage by reacting with free radicals, chelating free catalytic metals, and also by acting as oxygen scavengers.
The genus Globularia is commonly used in Moroccan folk medicine. Ethnomedicinal investigations have demonstrated that Globularia alypum , locally named ′Ain Larneb,′ is one of the most traditional plant remedies.[6] Its leaves are reported to be used in the treatment of diabetes and in renal and cardiovascular diseases.[6] They are also used as laxative, cholagogue, stomachic, purgative, sudorific,[7] antihyper-tensive,[8] and hypoglycemic.[9] The involvement of ROS in most of these disorders prompted us to investigate the antioxidant properties of G. alypum , which has not been explored until now.
MATERIALS AND METHODS
Plant material
Fresh G. alypum L. was collected during spring 2003 from the region of
Taza, Morocco. Taxonomic identification was performed by Dr. R. Tellal (Laboratoire
d′Ecologie Vιgιtale, Facultι des Sciences. El Jadida, Morocco).
A voucher specimen (KS2) is kept in the herbarium of the biology department.
Preparation of plant extract
Fresh aerial parts (stems and leaves) were washed and air-dried in shade
at room temperature. They were then mechanically powdered and sieved. One
hundred grams of the obtained fine powder was macerated in a hermetically
closed glass vessel for 48 h, at room temperature (25°C), under occasional shaking with 500 ml of a mixture of distilled water-methanol (3/2 v/v). The obtained crude preparation was centrifuged at 5000 g for 45 min (Sigma 2K15). After filtration, the supernatant filtrate was concentrated under reduced pressure at 25°C and the crude extract (10.75 g) stored at -20°C
until use.
Chemicals
Linoleic acid (99%), ethylenediaminetetra-acetic acid (EDTA), Tween
20 (polyoxyethylenesorbitan monolaurate), butylated hydroxytoluene (BHT)
and Folin-Ciocalteu reagents were purchased from Sigma. All other unlabelled
chemicals and reagents were of analytical grade.
Isolation of low-density lipoproteins
Human low-density lipoproteins (LDL) (1.019 <1.063) were isolated according
to
the method of Sattler,[10] using
a Beckman Optima TLX ultracentrifuge equipped with a TLA 100.4 rotor, in the
presence of EDTA (0.4 g/l). After separation, LDL was dialyzed overnight at 4°C
with 1/102 M sodium phosphate buffer (pH 7.0). For oxidation experiments,
the
LDL dialyzed solutions were adjusted by dilution to 100 µg/ml, and proteins
were measured by commercial assay (Pierce method, Rockford III, USA).
Measurement of oxygen consumption
Globularia alypum (Ga) extract (10 and 100 µg/ml) was added
to 1.5 ml of a 7.5 mM linoleic acid emulsion in 10 mM aqueous phosphate
buffer (pH 7.0) and 0.1% of Tween 20 (v/v) as emulsifier. The emulsion
was air saturated. A freshly prepared solution of CuSO4 was
added, at a final concentration of 10 µM, to initiate the oxidation
process. Measurement of the oxygen consumption, according to the modified
technique of Genot[11] using
Strathkelvin Instruments Oxymeter 949 was started by injection of the sample
into a thermostated (25.0±0.1°C) 2 ml measuring cell with
no headspace. Oxygen consumption was measured with a Clark electrode and
recorded for approximately 25 min at time intervals of 30 s with a computer-based
data collecting system. The oxymeter was standardized at 0% of c with
bisulfite sodium solution (3.85 M) and at 100% of O2 with air-saturated
water solution.
The oxygen consumption rate V (O2) in µM/l/s was calculated
by the following equation:
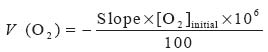
where the slope was calculated from the oxygen consumption curve,
[O2]initial = 2.6 10-4 M at 25°C Moller.[12]
The antioxidant index relative to the rate of oxygen consumption ( Ioxygen) was calculated by the following equation:[12]
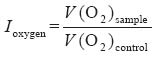
Measurement of conjugated dienes
The conjugated dienes (CD) were determined by measuring the absorbance at 234 nm according to the modified technique described by Esterbauer.[13] Briefly,
linoleic acid (7.5 mM) emulsified with Tween 20 (0.1%, v/v) or human LDL (100 µg/ml) mixture, in phosphate buffer (pH 7.0, 10 mM), was incubated alone (control) or with Ga extract (10 and 100 µg/ml). Oxidation was initiated by addition of freshly prepared CuSO4 (10 µM) and stopped by cooling in an ice bath in the presence of EDTA (100 µM) and BHT (20 µM). The absorbance readings were taken every 15 min over 270 min at 37°C
in a Beckman UV-Vis spectrophotometer.
The lipid peroxidation kinetic parameters: lag time (min), maximal
rate of oxidation (nM/min), and maximal amount of CD formation (µM)
were calculated using a molar extinction coefficient of 29 500/M/cm.
The inhibition of linoleic acid and LDL peroxidation was calculated by the following equation:
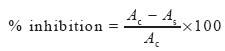
where Ac is the absorbance of the control reaction and As is the absorbance of the treated sample.
Determination of total polyphenolic compounds (PPC)
The amount of total PPC was measured by the method described by Taga[14] using
the Folin-Ciocalteu reagent. Briefly, samples and standards were prepared in
acidified (0.3% HCl) methanol-water solution (60/40 v/v). One hundred microliter of this preparation was added to 2 ml of 0.2% Na2CO3.
After 2 min, 100 µl of Folin-Ciocalteu/methanol (v/v) reagent was added to start reaction at room temperature (25°C)
during 30 min. The control mixture consisted of all reagents and solvent without
extract. The phenolic concentrations were expressed as phenol equivalent by comparison
with a standard calibration curve using phenol solution (0.01-1 mg/ml).
Statistical analysis
The results are presented as the mean±SD of five replicates. Data were analyzed using one-way analysis of variance (ANOVA) and group means were compared using Duncan′s
multiple range test. P values <0.05 were considered significant.
RESULTS Linoleic acid oxidation
As shown in [Figure - 1], Ga hydromethanolic extract exhibited a significant inhibition of linoleic acid oxidation as assessed by CD formation. The inhibition extents were 24% ( P <0.01) and 64% ( P <0.001), respectively, at 10 and 100 µg/ml, while BHT, used as standard antioxidant at 50 µg/ml, gave 76% ( P <0.001).
The kinetic parameters of oxidation [Table - 1] showed that at a low concentration (10 µg/ml), the plant extract did not influence the lag time and the maximal propagation rate of linoleic acid oxidation, but induced a low and significant reduction (24%) ( P <0.05) in the maximal amount of CD formation. At a high concentration (100 µg/ml), the extract caused a mean increase in lag time of 52±2.5 ( P <0.001) and inhibited both propagation rate (35%, P <0.001) and maximal amount of CD formation (64%, P <0.001).
The antioxidant properties of G. alypum were also demonstrated
by the oxygen consumption method where the plant extract induced significant
effect [Figure - 2]. Thus, the Ga extract (10 and 100 µg/ml) reduced the rate of oxygen consumption by 63% ( P <0.001) and 84% ( P <0.001), respectively. It must be noted that the effect observed with a concentration of 100 µg/ml was more efficient than that obtained with BHT at 50 µg/ml (77%, P < 0.001) [Table - 2].
Human LDL peroxidation
In order to confirm the protective action of Ga extract on linoleic acid oxidation, we tested its effect on human LDL oxidation by quantifying the CD formation.
The obtained results [Figure - 3] showed that copper-induced LDL peroxidation was significantly inhibited by Ga extract. The extent of inhibition of CD formation was 61%, ( P <0.001), 83% ( P <0.001) and 76% ( P <0.001), respectively, at 10 and 100 µg/ml of plant extract and BHT at 50 µg/ml.
The effects on kinetic parameters showed that the plant extract prolonged the lag time, diminished the propagation rate, and inhibited the maximal amount of CD formation [Table - 1]. Standard antioxidant BHT (50 µg/ml) did not affect the lag time but inhibited to a lesser extent the propagation rate and the maximal amount of CD formation.
DISCUSSION The present investigation carried out on the antioxidant properties of hydromethanolic extract of G. alypum clearly showed the protective activity on lipid peroxidation. However, as previously described,[15],[16] the efficiency of antioxidant activity was dependant on the used lipid system and the method of assessment.
Globally, in the presence of linoleic acid (simple lipid system), the antioxidant effect produced by the extract was lesser or higher than BHT, respectively, when assessed by CD formation or oxygen consumption. According to the kinetic parameters of linoleic acid oxidation, the plant extract (100 µg/ml) extended the lag time probably by increasing the initiation stage of the chain reaction. The plant extract also inhibited both the propagation rate and the maximal amount of CD formation.
After the CD formation, one of the first events of lipid peroxidation was the uptake of oxygen and formation of lipid peroxides.[17] In the presence of plant extract, linoleic acid oxidation showed significant decrease in the rate of oxygen uptake, which was higher than that induced by BHT. Probably, the plant extract reacts with peroxyl radicals inducing an inhibition of the lipid peroxidation chain propagation.[16]
The antioxidant effect exerted by the plant extract seems to be more efficient in protecting human LDL (complex lipid system) against peroxidation than linoleic acid. The extract increased the lag time and decreased the propagation rate and the maximal amount of CD formation, whereas BHT did not affect the lag phase and weakly decreased the propagation rate. Similar results were obtained with probucol[18] and with a synthesized antioxidant 4GBE43.[19]
Otherwise, the high amount of total phenolic content (120 mg of phenol equivalent per gram of extract) led us to suggest that these substances could be responsible of the antioxidant properties of the extract. Polyphenols were reported to have an important role in stabilizing lipid peroxidation[20] and are associated with a wide range of biological activities including antioxidant properties [21],[22],[23] due to their redox properties, as reducing agent or hydrogen atom donors.[4]
The implication of the polyphenolic compounds in the antioxidant activity of G. alypum is supported by previous results obtained with other Globularia species. Thus phenylethanoid glycosides extracted from Globularia Trichosantha ,[24] Globularia davisiana ,[25] and Globularitol obtained from Globularia orientalis[26] have been reported to possess scavenging properties.
In conclusion, the protective effect of Ga hydromethanolic extract toward linoleic acid emulsion and human LDL peroxidation was demonstrated by highly significant diminution of both oxygen consumption and CD formation. Further investigations are in progress to characterize the active compounds and to evaluate the usefulness in the treatment of disorders that involve oxidative stress.
REFERENCES
| 1. | Pryor WA. Oxidation and atherosclerosis. Free Radic Biol Med2000;28:1681-2. Back to cited text no. 1 |
| 2. | Sinclair AJ. Free radical mechanisms and vascular complications of diabetes mellitus. Diabetes Res 1993;2:7-10. Back to cited text no. 2 |
| 3. | Pryor WA. Cancer and free radicals. Basic Life Sci 1986;39:45-9. Back to cited text no. 3 [PUBMED] |
| 4. | Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem 2004;84:551-62. Back to cited text no. 4 |
| 5. | Aruoma O.I. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res 2003;523-4:9-20 Back to cited text no. 5 |
| 6. | Jouad H, Haloui M, Rhiouani H, El Hylali J, Eddouks M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in North centre region of Morocco (Fez-Boulman). J Ethnopharmacol 2001;77:175-82. Back to cited text no. 6 |
| 7. | Bellakhdar J. La pharmacopée marocaine traditionnelle. France: Ibis press; 1997. Back to cited text no. 7 |
| 8. | Ziyyat A, Legssyer H, Mekhfi H, Dassouli A, Serhouchni M, Benjelloun W. Phytotherapy of hypertension and diabetes in oriental Morocco. J Ethnopharmacol 1997;58:45-54. Back to cited text no. 8 |
| 9. | Jouad H, Maghrani M, Eddouks M. Hypoglycaemic effect of Rubus fructicosis L. and Globularia alypum L. in normal and streptozotocin-induced diabetic rats. J Ethnopharmacol 2002;81:351-6. Back to cited text no. 9 [PUBMED] [FULLTEXT] |
| 10. | Sattler W, Mohr D, Stocker R. Rapid isolation of lipoproteins and assessment of their peroxidation by high performance chromatography post-column chemiluminescence. Methods in Enzymol 1994;233:469-89. Back to cited text no. 10 [PUBMED] |
| 11. | Genot, C, Kansci G, Laroche M. Measurement of phospholipid oxidation in model membranes by determination of oxygen consumption with a semi-automatic polarographic method. Sci des Aliments 1994;14:673-82. Back to cited text no. 11 |
| 12. | Moller JKS, Madsen HL, Aaltonen T, Skibsted LH. Dittany (Origanum dictammus) as a source of water-extractable antioxidants. Food Chem 1999;64:215-19. Back to cited text no. 12 |
| 13. | Esterbauer H, Streigl G, Puhl H, Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun 1989;6:67-75. Back to cited text no. 13 |
| 14. | Taga MS, Miller EE, Pratt DE. Chia seeds as a source of natural lipid antioxidant. J Am Oil Chem Soc 1984;61:928-31. Back to cited text no. 14 |
| 15. | Takaya Y, Kondo Y, Furukawa T, Niwa M. Antioxidant constituents of Radish Sprout (Kaiware-daikon), Raphanus sativus L. J Agric Food Chem 2003;5:8061-6. Back to cited text no. 15 |
| 16. | Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem 2004;85:633-40. Back to cited text no. 16 |
| 17. | Burton GW, Ingold KU. Vitamin E: Application of the principles of physical organic chemistry to the exploration of its structure and function. Acc Chem Res1986;19:194-201. Back to cited text no. 17 |
| 18. | Gotoh N, Shimizu K, Komuro E, Tsuchiya J, Noguchi N, Niki E. Antioxidant activities of probucol against lipid peroxidations. Biochim Biophys Acta 1992;1128:147-54. Back to cited text no. 18 [PUBMED] |
| 19. | Ezure T, Kanayama T, Nishino C. Action of the novel antioxidants 4GBE43 and 2BBE43 against lipid peroxidation. Biochem Pharmacol 2001;62:335-40. Back to cited text no. 19 [PUBMED] [FULLTEXT] |
| 20. | Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of harnjyur (Chrysanthemum morifolium Ramat). Lebensmittel-Wissenschaft und Technologie 1999;32:269-77. Back to cited text no. 20 |
| 21. | Vinson JA, Dabbagh YA, Serry MM, Jang J. Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart diseases. J Agric Food Chem 1995;43:2800-2. Back to cited text no. 21 |
| 22. | Roginsky V. Chain-breaking antioxidant activity of natural polyphenols as determined during the chain oxidation of methyl linoleate in Triton x-100 micelles. Arch Biochem Biophys 2003;414:261-70. Back to cited text no. 22 [PUBMED] [FULLTEXT] |
| 23. | Hou L, Zhou B, Yang L, Liu ZL. Inhibition of human low density lipoprotein oxidation by flavonols and their glycosides. Chem Phys Lipids 2004;129:209-19. Back to cited text no. 23 [PUBMED] [FULLTEXT] |
| 24. | Çalis I, Kirmizibekmez H, Sticher O. Iridoid glycosides from Globularia trichosantha. J Nat Products 2001;64:60-4. Back to cited text no. 24 |
| 25. | Çalis I, Kirmizibekmez H, Tasdemir D, Ireland CM. Iridoid glycosides from Globularia davisiana. Chem Pharm Bull 2002;50:678-80. Back to cited text no. 25 |
| 26. | Çalis I, Kirmizibekmez H, Tasdemir D, Sticher O, Ireland CM. Sugar esters from Globularia orientalis. Z Naturforsch 2002;57:591-6. Back to cited text no. 26 |
Copyright 2005 - Indian Journal of Pharmacology
The following images related to this document are available:
Photo images
[ph05058t1.jpg]
[ph05058t4.jpg]
[ph05058f2.jpg]
[ph05058t2.jpg]
[ph05058t5.jpg]
[ph05058f1.jpg]
[ph05058t3.jpg]
[ph05058f3.jpg]
|
