
|
Iranian Journal of Environmental Health, Science and Engineering
Iranian Association of Environmental Health (IAEH)
ISSN: 1735-1979
Vol. 4, Num. 2, 2007, pp. 127-132
|
Untitled Document
Iranian Journal of Environmental Health Science & Engineering,Vol.
4, No. 2, 2007, pp. 127-132
PERFORMANCE EVALUATION OF ELECTROCOAGULATION PROCESS FOR
DIAZINON REMOVAL FROM AQUEOUS ENVIRONMENTS BY USING IRON ELECTRODES
1E. Bazrafshan, *1A. H. Mahvi, 1S. Nasseri, 2M. Shaieghi
1Center for Environmental Researches and Department of Environmental Health Engineering, School of Public Health,
Medical Sciences/University of Tehran, Tehran, Iran
2Department of Medical Entomology and Vector Control, School of Public Health, Me dical Sciences/University
of Tehran, Tehran, Iran
*Corresponding author-Email: ahmahvi@yahoo.com Tel: +98 21 8895 4914,
Fax: +98 21 8895 0188
Received 18 January 2007; revised 26 February 2007; accepted 25 March 2007
Code Number: se07019
ABSTRACT
The present study investigates the removal of pesticide by electrocoagulation
process. A glass tank in 1.56 L volume with four iron plate electrodes was
used to perform the experiments. The electrodes connected to a
DC power supply (bipolar mode). The tank was filled with synthetic wastewater
were which contained
diazinon pesticide in concentration of 10, 50 and 100 mg/L. The percent of
diazinon removal was measured
at pH= 3, 7 and 10 and in electric potential range of 20-40V by thin layer
chromatography method. The
results indicated that initial concentration of diazinon can affect efficiency
removal and for higher
concentrations of diazinon, higher electrical potential or more reaction time
is needed. The results showed
that for a given time, the removal efficiency increased significantly with
increase of voltage. The highest
electrical potential (40V) produced the quickest treatment with >99% diazinon
reduction occurring after 60 min. The final pH for iron electrodes was always
higher than initial pH. Finally it can be concluded that
electrocoagulation process (using iron electrodes) is a reliable, efficient
and cost-effective method for
removal of diazinon from aqueous environments, especially designed for pH=3 and
voltage=40V.
Key words: Pesticide, diazinon, electrocoagulation, aqueous environments
INTRODUCTION
Pesticides comprise a variety of toxic
substances and are used in agriculture as well as indoors
to kill pests. The health risk of pesticides to
humans is worsened by the fact that many of these substances have been shown to be
carcinogenic and mutagenic. Organophosphorus
pesticides (OPPs) have a higher acute toxicity
than organochlorines, but they have the advantage
of being rapidly degraded in the environment
(Zohair, 2001; Legrouria et al., 2005). Organophosphorus pesticides are widely found in water
resources. They are released into the environment
from manufacturing, transportation and agriculture applications (Honeycutt and Schabcker.,
1994). Several investigators found high levels of
pesticide residues after the washing and/or safety
period (Kariem et al., 1991; Ramadan et
al., 1992; Saleh et al., 1993). Organophosphorus pesticides
are very toxic when absorbed by human organisms because of acetyl-cholinesterase de
activation (Berijani et al., 2006). The European Union
(EU) allows a maximum concentration of 0.1 ìg/L
of each individual pesticide and 0.5 ìg/L of the
sum of pesticides in drinking water (European
Union., 1998). Diazinon is one of the most widely
used organophosphate insecticides in agriculture (Gokcimen et al., 2007). Chemical formula of diazinon is
C12H21N2O3 PS, molecular weight is 304.3 and density (at
20oC) is 1.117 g/mL (Badawya et
al., 2006). It is an organophosphorus insecticide classified by the World
Health Organization (WHO) as "moderately
hazardous" class II. It was associated with toxicity to
aquatic organisms at concentration of 350 ng/L, with
an LC50 in killifish (48 h) of 4.4 mg/L. Fetal
human doses were found to be in the range from 90 to 444 mg/kg (Shemer and Linden, 2006).
Toxic effects of diazinon are attributed to its
inhibition of the enzyme acetyl-cholinesterase. Diazinon
is relatively water soluble (40 mg/L at 25
oC), non-polar, moderately mobile and persistent in
soil; hence, it is of concern for groundwater and
surface derived drinking water (Kidd and James,
1991). Diazinon has a log Kow of 3.3, vapor pressure
of 1.4×10-4 mm Hg at 20 oC, and Henry's law constant of
1.4×10-6 atm m3/mol which
would indicate that it would not easily volatilize from
soil or water. However, vaporization of diazinon
from water of up to 50% of applied mass was
reported (Howard, 1991). Diazinon and its metabolites
have been detected in aquatic systems worldwide (Shemer and Linden, 2006). Its
sediment-water partition coefficient is small indicating
minor adsorption of the substance on sediments. Contamination of water by pesticides is mainly
due to runoff, usually within a few weeks after application. Once in the environment, its
fate depends on volatilization, hydrolysis, and
photolysis (Feigenbrugel et al., 2004). Diazinon
undergoes fast hydrolysis at acidic and basic conditions,
with half lives of 0.5, 171, and 6 days at pH= 3.1,
7.3, and 10.4 (at 2025°C), respectively (Zhang
and Pehkonen, 1999; Mansour et al., 1999).
The pesticide and chemical industries
are considered to generate wastewaters containing toxic and non-biodegradable compounds
that remain in the environment even after their wastewaters have been subjected to
conventional processing (Badawy, 1998; Jannsens et al., 1997). Therefore, the human population is exposed
to pesticides and other organic micropollutants
either through drinking water or via the food supply.
In addition, there is a formation of mutagenic compounds during conventional
oxidation processes (Jannsens et al., 1997; Bertanza et al., 2001). Therefore, it is very important to
develop water and wastewater treatment technologies
for the removal of toxic and refractory organic compounds from water and wastewater. A
way for diazinon removal from aqueous environments is electrocoagulation process which is without
any chemical material using and only uses
transferring iron ion's electron to solution in the form of
metal. In this method, diazinon precipitates and remove.
In fact, in electrocoagulation process,
the flocculating agent is generated by electro
oxidation of a sacrificial anode, generally made of iron
or aluminum. In this process, the treatment is
done without adding any chemical coagulant or
flocculants, thus reducing the amount of sludge which must
be disposed (Cenkin and Belevstev, 1985). An examination of the chemical reactions occurring
in the electrocoagulation process shows that the
main reactions occurring at the electrodes are:
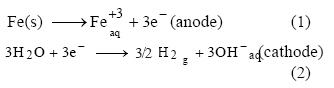
In addition, Fe3+ and
OH- ions generated at electrode surfaces react in the bulk
wastewater to form ferric hydroxide:
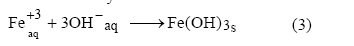
The iron hydroxide flocs act as adsorbents
and/or traps for pollutants and so eliminate them
from the solution (Cenkin and Belevstev, 1985; Ogutveren et al., 1994).
The objective of this research was to survey
efficiency of electrocoagulation process removal of diazinon from aqueous environments with
iron electrodes and determination of the effects
of voltage, pH and reaction time on the removal efficiency.
MATERIALS AND METHODS
This study has been conducted in
the environmental chemistry laboratory of School
of Public Health at Tehran University of Medical Sciences, in late 2006. All chemicals
including diazinon, acetone, hexane, sodium hydroxide
pellets, concentrated sulfuric acid and potassium
chloride were provided from Merck Company. Desired concentrations of diazinon solution were
prepared by mixing proper amount of diazinon (60%)
with deionized water. In order to increase the conductivity of the solution to 1.6
mS/cm, potassium chloride (1 N) was added to the
solution before injecting it into the apparatus. The
chloride salt added to the solution can also prevent
the formation of the oxide layer on the anode and therefore reduce the passivation problem of
the electrodes. The pH of the initial solution was adjusted by using sulfuric acid solution and
sodium hydroxide (0.1 M). Experiments were
performed in a bipolar batch reactor, with four iron
electrodes connected in parallel (bipolar mode). Only
the outer electrodes were connected to the power source, and anodic and cathodic
reactions occurred on each surface of the inner
electrode when the current passed through the
electrodes. The internal size of the cell was 10 cm×13cm×12cm (width×length×depth) with
an effective volume of 1000 cm3. The volume (V)
of the solution of each batch was 1 l. The active
area of each electrode was 10×10 cm. The
distance between the electrodes was 1.5 cm. A
power supply pack having an input of 220V and
variable output of 040V with maximum current of 5
am was used as a direct current source. The pH
values in influent and reactor unit were measured
using a pH meter model E520 (Metrohm Herisau, Switzerland). A Jenway conductivity meter
(Model 4200) was employed to determine the
conductivity of the solution. Samples were extracted after
10, 20, 40 and 60 minutes. Diazinon concentration
was determined with mobile phase of hexane-acetone (10:40) in water and absorbance detection at
254 nm. Finally diazinon concentration was
determined by high performance thin layer
chromatography method (TLC scanner 3, program cats 4,
Camay, Swiss). In order to study the effect of turbidity
on diazinon pesticide removal by
electrocoagulation process, a set of experiments with different
initial concentrations of turbidity (10, 50 and 200
NTU) were performed under optimum conditions
(pH=3, time=60 min, voltage= 40 V). Standard solution
of turbidity was prepared by dissolving 5 g of
hydrazine sulfate and 50 g of hexamethylenetetramine in
one liter of distilled water (this solution is equal to
4000 NTU). Also, in order to study the effect of
organic matter presence (such as COD) on diazinon removal by electrocoagulation process, a set
of experiments with different initial concentrations
of COD (100, 500 and 1000 mg/L) were performed under optimum conditions (pH=3, time=60
min, voltage= 40V). Standard solution of COD was prepared by dissolving 8.502 g of
potassium hydrogen phthalate
(KC8H5O4) in distilled
water and diluted to 1,000 mL. The prepared solution
had a theoretical COD value of 10,000 mg/L.
RESULTS
The electrocoagulation process is quite
complex and may be affected by several operating parameters such as pollutant concentration,
initial pH, electric potential (voltage), presence of
organic matter (such as COD), electrical conductivity
and turbidity. The results of diazinon pesticide
removal for various initial concentrations of 10, 50 and
100 mg/L by electrocoagulation process using iron electrodes are shown in Table 1, 2 and 3.
Results illustrate that diazinon removal
efficiency for different conditions such as initial pH
and electrical potential is significant (>90%). Table 4
illustrates the results of consumed iron
electrode during electrocoagulation process for various
pH and diazinon initial concentrations. With
increase in electrical potential, the amount of
electrode consumption increased, too. So, the
highest electrode consumption was observed at
electrical potential of 40V. Table 5 shows the amount
of consumed energy during electrocoagulation process at voltage 40V and for different
initial concentrations of diazinon. These results
illustrated that consumed energy decreased with increase
in diazinon initial concentration.
DISCUSSION
In the present study, electrocoagulation
process has been evaluated as a treatment technology
for diazinon removal from industrial effluents.
Diazinon removal efficiency at different condition
(pH, electrical potential) in various times was
evaluated. It has been established in previous studies (Vik et al., 1984; Chen et al., 2000) that pH has
a considerable effect on the efficiency of the electrocoagulation process. Also, as observed
by other investigators the pH of the medium
changed during the process. This change depends on
the type of electrode material and initial pH. In
this study, the pH was varied in the range 310 in
an attempt to investigate the influence of this parameter on the removal of diazinon.
Removal efficiencies of diazinon as a function of initial
pH with iron electrodes are presented in Table 1
and 3. As observed by other investigators (Vik et
al., 1984), a pH increase occurs when the initial pH
is low (< 7). Vik ascribed this increase to
hydrogen evolution at cathodes. In addition, if the initial
pH is acidic, reactions would shift towards which causes a pH increase. In alkaline medium (pH
> 8), the final pH does not vary very much and
a slight drop was recorded. This result is in
accord with previously published works and suggests
that electrocoagulation can act as pH buffer. In
this research, the initial pH did not affect the
removal efficiencies significantly over a wide
range. Therefore, pH adjustment before treatment is
not required in practical applications. The
highest efficiency of diazinon removal observed in
acidic medium (pH=3). The pH variation of solution
after electrocoagulation process in various
voltages showed that the final pH for all of
experiments with iron electrodes is higher than initial
pH, which is in agreement with other studing
(Kobya et al., 2003).
Preliminary laboratory testing of the
electrolysis cell involved determining the effect of
applied voltage on the efficiency of diazinon removal. It
is well-known that electrical potential not only determines the coagulant dosage rate but also
the bubble production rate and size and the flocs growth (Letterman et al., 1999; Holt et al., 2002), which can influence the treatment efficiency
of the electrocoagulation. Therefore, the effect
of electrical potential on the pollutants removal
was investigated. As expected, it appears that for
a given time, the removal efficiency increased significantly with increase of electrical
potential. The highest electrical potential (40V)
produced the quickest treatment with >90%
diazinon reduction occurring after 60 min and the
lowest diazinon removal efficiency occurred in the
lowest electrical potential (20V). This is ascribed to
the fact that at high voltage, the amount of iron oxidized increased, resulting in a greater
amount of precipitate for the removal of pollutants.
In addition, it was demonstrated that bubbles
density increases and their size decreases with
increasing current density (Khosla et al., 1991), resulting
in a greater upwards flux and a faster removal of pollutants and sludge flotation. As the
current decreased, the time needed to achieve
similar efficiencies increased and the results of
this research confirm this fact. This expected
behavior is explained by the fact that the
treatment efficiency was mainly affected by charge
loading (Q=It), as reported by Chen (Chen et
al., 2000).
However, the cost of the process is
determined by the consumption of the sacrificial
electrode and the electrical energy which economically
are the advantages of this method. These results suggest 40V as an optimal electrical potential
for the treatment of effluents containing
diazinon, since it ensures the quickest removal rate
with the lowest cost.
A set of experiments was performed with
different initial concentrations of diazinon to determine
the time required for removal under various
conditions of electrocoagulation process. The results
obtained at different electrical potential showed that
initial concentration of diazinon can effect on
efficiency removal and for higher concentration of
diazinon, higher electrical potential or more reaction time
is needed. On the other hand, if the initial concentration increases, the time required
of process should increase too. And, also it is
clear from Table 1 and 3 that for higher
concentrations, greater time was needed for removal of
diazinon, but that higher initial concentrations of
diazinon were reduced significantly in relatively less
time than lower concentrations. The time taken for reduction thus increased slowly with increase
in concentration. This can be explained by the
theory of dilute solution. In dilute solution, formation
of the diffusion layer at the vicinity of the
electrode causes a slower reaction rate, but in
concentrated solution the diffusion layer has no effect on
the rate of diffusion or migration of metal ions to
the electrode surface (Chaudhary et al., 2003).
The time dependence of diazinon removal
by electrocoagulation process at different pH
shown in Tables 1 and 3. describe that up to 30-70 %
of the initial concentration decreased within 20
min of the process at different concentrations and
the residual diazinon concentration in effluent
were less 0.01 mg/L and finally at the end of
reaction time (60 min) reached to zero mg/L so we
could discharge treated effluents to environment,
in safety. At the beginning of process the
diazinon removal was rapid and later it decreased
gradually over almost the entire process examined.
Diazinon was more abundant at the beginning
of the electrocoagulation process, and the
generated iron hydroxides due to corrosion of the anode
at that time will form complexes with diazinon and therefore rapid removal of diazinon was
observed. A set of experiments was performed with
different initial concentrations of diazinon (10, 50 and
100 mg/L) to examine the effect of the presence of organic matter (such as COD with
concentration 100, 500 and 1000 mg/L O2) and various levels
of turbidity (10, 50 and 200 NTU) in wastewater on the removal efficiency of diazinon. The
results obtained at optimum conditions (pH=3,
reaction time=60 min and voltage=40V) showed that
the removal efficiency for various concentrations
of diazinon decreased, but it was not significant. Hence the electrocoagulation process can
be efficiently applied for diazinon removal in
presence of organic matter and turbidity.
A series of tests were conducted with
different concentrations of diazinon in the solution and
the weight of the electrode consumed with respect
to different voltage levels (given in Table 4)
show that the higher the applied voltage, the higher
the weight of the electrode consumed. Also, higher initial diazinon concentrations in the solution
result in higher electrode consumption weights. As
the Table illustrates, the weight of electrode
consumed at 40 volts was much higher that that at 20
volts. Also, an increase in initial diazinon
concentration to 500 mg/L, did not result in an significant
increase in electrode consumption. However, at
this concentration, coagulation has taken place and
the high formation of the flocs has helped the complexation of diazinon and there was no
need for much consumption of the electrode as at
low initial diazinon concentrations.
Table 5 shows energy consumption results for
the removal of one gram of diazinon at 40V,
initial diazinon concentrations of 5, 50 and 500
mg/L, and pH 3, 7 and 10. It can be concluded that
the consumed energy decreased with increase in
initial diazinon concentration, because the flocs
formation will help the adsorption of the diazinon from
the solution. Finally, it can be concluded that electrocoagulation method
is a reliable, safety, efficient and cost-effective method for
removal of diazinon from aqueous environments,
especially designed for pH=3 and voltage=40V. On the
other hand, in this study it was shown that electrocoagulation process using
iron electrodes achieves a fast and effective reduction of diazinon (more
than 99%) present in industrial
effluents. Indeed, the reported results show that electrocoagulation is faster
and more effective process as compared to other methods
alone. Nevertheless, further studies should be carried
out to confirm the practical feasibility of this
method for treating various wastewaters and with
different condition.
ACKNOWLEDGEMENTS
This research was financially supported by
Center for Environmental Research, Tehran
University of Medical Sciences: project # 2711.
REFERENCES
- Badawy, M. I., (1998). Use and impact of pesticides
in Egypt. Int. Environ. Health Res., 8: 223240.
- Berijani, S., Assadi, Y., Anbia, M., Milani Hosseini, M.
R., Aghaee, E., (2006). Dispersive liquidliquid
microextraction combined with gas chromatography-flame
photometric detection Very simple, rapid and sensitive method for
the determination of organophosphorus pesticides in
water. J. Chromatography., 1123: 19.
- Bertanza, G., Collivignarell, C., Pedrazzen, I. R.,
(2001). The role of chemical oxidation in combined
chemical-physical and biological processes: experience of
industrial wastewater treatment. Water. Sci. Technol., 44: 109116.
- Cenkin, V. E., Belevstev, A. N., (1985).
Electrochemical treatment of industrial wastewater, Eff. Water. Treat.
J., 25 (7): 243249.
- Cenkin, V. E., Belevstev, A. N., (1985).
Electrochemical treatment of industrial wastewater, Eff. Water. Treat.
J., 25 (7): 243249.
- Chaudhary, A. J., Goswami, N. C., and Grimes, S. M.,
(2003). Electrolytic removal of hexavalent chromium
from aqueous solutions. J. Chem. Technol. Biot., 78: 877883.
- Chen, X., Chen, G., Po, L. Y., (2000). Separation
of pollutants from restaurant wastewater by electrocoagulation. Sep. Purif.
Technol., 19: 6576.
- Drinking Water Guideline, 98/83/EEC, European
Union, Brussels, 1998.
- Feigenbrugel, V., Le Calve, S., Mirabel, P.,
(2004). Temperature dependence of Henry's law constants
of metolachlor and diazinon, Chemosphere. 57, 319327.
- Gokcimen, A., Gulle, K., Demirin, H., Bayram, D.,
Kocak, A., Altuntas, I., (2007). Effects of diazinon at
different doses on rat liver and pancreas tissues. Pestic.
Biochem. Phys., 87: 103108.
- Holt, P. H., Barton, G. W., Wark, M., Mitchell, A. A.,
(2002). A quantitative comparison between chemical dosing
and electrocoagulation, Colloids Surf. A: Physicochem.
Eng. Aspects., 211: 233248.
- Honeycutt, R. C., Schabcker, D. J., (1994). Mechanisms of
- Pesticides Movement into Ground Water, CRC Press,
Boca Raton, FL.
- Howard, P., (1991). Handbook of Environmental Fate
and Exposure Data for Organic Chemicals, Lewis
Publishers, Inc., Chelsea, MI. 209221.
- Jannsens, I., Tanghe, T., Verstraate, W.,
(1997). Micropollutants: A Bottleneck in sustainable
wastewater treatment. Water. Sci. Technol., 35: 1326.
- Kariem, A. M., Ramadan, R. A., Mostafa, A. M.,
(1991). Fungicides in controlling strawberry fruit and their
residues in fruits. Egyptian. J. Appl. Sci. 6: 710719.
- Khosla, N. K., Venkachalam, S., Sonrasundaram, P.,
(1991). Pulsed electrogeneration of bubbles for
electroflotation. J. Appl. Electrochem., 21: 986990.
- Kidd, H., James, D. R., (1991). The
Agrochemicals Handbook, 3rd Ed. Royal Society of Chemistry
Information Services, Cambridge, UK., 514.
- Kobya, M., Taner-Can, O., and Bayramoglu, M.,
(2003). Treatment of textile wastewaters by
electrocoagulation using iron and aluminum electrodes. Journal of
Hazardous Materials B100., 163178.
- Legrouria, A., Lakraimib, M., Barrougb, A., De Royc,
A., Bessec, J. P., (2005). Removal of the herbicide 2,
4-dichlorophenoxyacetate from water to
zincaluminiumchloride layered double hydroxides, Water. Res., 39:
34413448.
- Letterman, R. D., Amirtharajah, A., and O'Melia, C.
R., (1999). A Handbook of Community Water Supplies.
5th Ed. AWWA, Mc Graw-Hill, N. Y. USA.
- Mansour, M., Feicht, E. A., Behechti, A., Schramm,
K.W., Kettrup, A., (1999). Determination photostability
of selected agrochemicals in water and soil,
Chemosphere 39: 575585.
- Ogutveren, U. B., Gonen, N., Koparal, A. S. (1994).
Removal of chromium from aqueous solutions and plating
bath rinse by an electrochemical method, Int. J. Environ.
Stud., 45: 8187.
- Ramadan, R. A., Saleh, M. A., El-Shemy, M. K.,
(1992). Residues of some pesticides on cucumber, green
pepper and eggplant fruits under plastic green house
conditions., Egyptian. J. Appl. Sci., 7: 861870.
- Saleh, M. A., El-Shemy, M. K., Flaifel, M. A.,
(1993). Determination of benomyl, dichlorfluanid and
prodione residues in strawberry fruits. Egyptian. J. Appl.
Sci., 8: 1520.
- Shemer, H., Linden, K. G., (2006). Degradation and
by-product formation of diazinon in water during UV
and UV/H2O2 treatment. J. Hazardous. Mater. B136,
553559.
- Vik, E. A., Carlson, D. A., Eikum, A. S., Gjessing, E.
T., (1984). Electrocoagulation of potable water. Water.
Res., 18: 13551360.
- Zhang, Q., Pehkonen, S. O., (1999). Oxidation of
diazinon by aqueous chlorine: kinetics, mechanisms, and
product studies, J. Agric. Food. Chem., 47: 17601766.
- Zohair, A., (2001). Behaviour of some
organophosphorus and organochlorine pesticides in potatoes during
soaking in different solutions. Food. Chemical.
Toxicol., 39: 751755. 4413448.
© 2007 Tehran University of Medical Sciences Publications
The following images related to this document are available:
Photo images
[se07019t4.jpg]
[se07019t3.jpg]
[se07019t1.jpg]
[se07019t2.jpg]
[se07019t5.jpg]
|
