 |
| About Bioline | All Journals | Testimonials | Membership | News |
|
||||||
|
||||||
Iranian Journal of Environmental Health, Science and Engineering, Vol. 7, No. 3, 2010, pp. 241-252 Methyl Tert-Butyl Ether Adsorption On Surfactant Modified Natural Zeolites 1S. K. Ghadiri, 1R. Nabizadeh, *1, 2A. H. Mahvi, 1S. Nasseri, 3, 4H. Kazemian, 1A. R. Mesdaghinia, 1Sh. Nazmara 1 Department of Environmental Health Engineering, School of Public Health, and Center for Environmental Research, Tehran University of Medical Sciences, Tehran, Iran *Corresponding author: E-mail: ahmahvi@yahoo.com Tel: 0912 32118 27, Fax: 021 889 50 188 Reseived 19 May 2010; revised 20 June 2010; accepted 1 July 2010 Code Number: se10027 ABSTRACT Surfactant-modified clinoptilolite-rich tuff was used for the removal of methyl tert-butyl ether (MTBE) from aqueous solutions. Clinoptilolite zeolite from Miyaneh region of Iran was treated with sodium chloride and then modified with hexadecyltrimethylammonium chloride (HDTMA-Cl) and n-Cetylpyridinium bromide (CPB) to be used in different experimental conditions. The ability of raw or Non-Modified Zeolite (NMZ) and Surfactant-Modified Zeolites (SMZ) to remove MTBE from aqueous solutions was investigated as a function of contact time, pH and concentrations of adsorbent and adsorbate, by using a batch technique in aqueous system. The removal of MTBE from aqueous solutions by modified zeolites seemed to be more effective than non-modified samples. Also, HDTMA-modified zeolite had more effective performance than CPB-modified zeolite. The adsorption efficiency of MTBE onto SMZS was found to increase by contact time and adsorbent concentrations, and by decreasing of pH and adsorbate concentrations. Empirical adsorption models of Langmuir and Freundlich were applied for the experimental data. Results showed that Langmuir isotherm was more suitable for this process. The experimental data fitted very well with the pseudo-second-order kinetic model. It was overally found that Surfactant-Modified Zeolites is an effective adsorbent for removal of methyl tert-butyl ether from contaminated solutions. Key words: Clinoptilolite; Surfactant Modified Zeolites; Methyl Tert-Butyl Ether; Adsorption INTRODUCTION Methyl tert–butyl ether (MTBE) is an anti-knocking agent that has been added to gasoline since 1970s and replaced with tetraethyllead to promote combustion efficiency and also to reduce air pollution (Mortazavi et al., 2005). Due to its high water solubility (43,000–54,300 mg/L) and low Henry’s law constant (0.023–0.12; dimensionless), MTBE is mobile and persistent in the environment (Mackay et al., 1993). However, with the widespread use of MTBE, it has become one of the most frequently detected underground water pollutants (Klinger et al., 2004; Schmidt et al., 2004). For example MTBE concentration exceeded 1000 mg/L in 63% of 445 sites sampled in California, Texas and Maryland (Rubin and Ramaswami, 2001) caused by gasoline leaking from underground storage tanks, pipelines and other components of gasoline distribution systems (Deeb et al., 2003). The use of MTBE has been banned as gasoline additive in the USA and Europe since 2003 (Atienza et al., 2005) because of its carcinogenicity (US-EPA, 1998). In contrast to USA, fuel oxygenates are still in use as octane enhancers at large amounts (about 6.9% – 10%) in Iran. In a research in Iran, out of 68 groundwater wells that were located near different gasoline stations, 8 wells were identified contaminated by MTBE at concentrations between 0.3 and 1.7 mg/L (Kaykhaii and Mirbaloochzahi, 2008). In another research, in samples obtained from the drainage water of a gasoline station in Zanjan city, the concentration of MTBE was approximately 100 mg/L (Eslami et al., 2008). Several methods have been proposed and tested for MTBE removal from water in Iran such as air stripping (Soltanali and Hagani, 2008), photocatalytic degradation (Eslami et al., 2008), degradation of MTBE by fenton reagent (Khavanin et al., 2005) and biodegradation (Alimmohamadi et al., 2005). However its resistance to chemical oxidation and biodegradation, leads to the formation of byproducts such as tertiary-butyl alcohol (TBA)(Liang et al., 1999; Leitner et al., 1994; Mormile et al., 1994). Since TBA is more polar and has higher Henry’s law constant, its removal from water is even more difficult than MTBE (Flores et al., 2000). Other byproducts such as bromate may be also produced from oxidation processes (Acero et al., 2001). Among the MTBE removal processes, adsorption is believed to be a simple and effective method for water and wastewater treatment. In addition, absence of undesirable byproducts in drinking water is the advantage of using adsorption processes for removal of MTBE (Quinlivan et al., 2005). For this reason, the success of the adsorption technique largely depends on the development of an efficient adsorbent. Natural zeolites are umpteenth, available and inexpensive resources, which are characterized by high cation exchange capacities (CEC), high surface areas and cage-like structures that can be found in many areas of the world. Due to their high cation-exchange capacity, natural zeolites have been widely used as adsorbents in separation and purification processes in the past decades. There are more than 40 different types of natural zeolites around the world. Among the natural zeolites, clinoptilolite (Heulandite-type zeolite) is the most abundant natural zeolite and is widely used in the world as adsorbents for adsorption of ions and organic materials in wastewater treatment processes ( Díaz-Nava et al., 2009; Wang and Peng, 2010). According to the published results, zeolites with high-silica perform a very large single-solute MTBE adsorption uptake which is even more than activated carbon (Senatalar et al., 2004; Bi et al., 2005; Hung and Lin, 2006). Zeolites that can exist naturally and synthetically, have the adsorption capability for water pollutants. The presence of abundant natural zeolite minerals in Iran can play a great role to develop this technology. Data from published researches show that, clinoptilolite is a good adsorbent for different polar and non-polar molecules such as aliphatic hydrocarbons, aromatic hydrocarbons, alcohols, ketons, CO2, SO2, NO2, NO, H2S, NH3, and other similar molecules (Malherbe et al., 1995). Due to the permanent negative charge on the crystal structures of zeolites, raw natural zeolites usually have little or no desire for anions and perform low adsorption for organic materials in aqueous solution (Alkaram et al., 2009). To increase the ability of zeolites to remove anions and organic water pollutants, it is necessary to modify their surfaces (Díaz-Nava et al., 2009). Modification of natural zeolites and altering its surface chemistry can be done in several methods such as acid treatment, ion exchange, and surfactant treatment, making the modified zeolites suitable for adsorption of anions and organic materials. Modification of zeolites with surfactants to remove multiple types of contaminants from water is a common method. The different types of surfactants which have been used for surfactant modified zeolite (SMZ) production include: tetramethylammonium, cetyltrimethylammonium (CTMA), hexadecyltrimethylammonium (HDTMA), octadecyldimethyl benzyl ammonium (ODMBA), n-cetylpyridinium(CPD), benzyltetradecyl ammonium (BDTDA), stearyldimethylbenzyl ammonium (SDBAC), N,N,N,N_,N_,N_-hexamethyl-1,9- nonanediammonium, and polyhexamethylene-guanidine (Wang and Peng, 2010). Researchers concluded that SMZ is an effective adsorbent for removal of petroleum compounds from aqueous solutions and SMZ can absorb petroleum compounds by a partitioning mechanism and additionally SMZ can be regenerated easily (Altare et al., 2007; Torabian et al., 2010). The main goal of this study was to survey the effect of different surfactants (i. e., HDTMA and CPB) on the adsorption characteristics of surface modified natural zeolite (i. e., clinoptilolite from the Miyaneh region, Iran) for the separation of methyl tert-butyl ether from contaminated water. The effects of pH, initial concentration of MTBE, contact time and adsorbent dosage on the adsorption performance were investigated. The adsorption isotherm and kinetic of MTBE on surfactant-modified zeolites in an aqueous system and batch adsorption technique were also investigated. MTBE removal was selected in this paper due to its impact on groundwater and clinoptilolite zeolite was selected due to its high potential in adsorption of soluble pollutants. MATERIALS AND METHODS Adsorbate and adsorbent Methyl tert-butyl ether (C5H12O; mw=88.15), with >99 % purity (HPLC grade), supplied by Merck company, was used as adsorbate. The adsorbent used in this study, clinoptilolite-rich tuff, was supplied from Miyaneh region (Azarbayjan province, North West of Iran). The clinoptilolite-rich tuff was milled and sieved to 0.21- 0.25mm (ASTM sieve size no. 70 to 60), and then washed thoroughly several times with tap water to wash out any mud and dusts, and then saturated in deionized water for 24 h for dissolution of salts. Samples were then dried at 250ºC in oven for 24 h to remove any organic materials (Koh and Dixon, 2001). Clinoptilolite-rich tuff properties were determined by X-ray fluorescence (XRF) (Oxford, ED2000) and X-ray diffractometer (XRD, model: XD-5A, Shimadzu). For surface morphology investigation of the zeolites, scanning electron microscopy (SEM, model: XL-30, Philips) was used (Torabian et al., 2010). In addition, the total CEC and external CEC (ECEC) of Miyaneh clinoptilolite-rich tuff was measured by Haggerty and Bowman method (Haggerty and Bowman, 1994). Modification of Miyaneh clinoptilolite-rich tuff converting to cationic form In order to saturate the zeolite adsorption sites with sodium, 10 g of zeolite was added to a bottle with 100 mL sodium chloride aqueous solutions of 2M concentration. Then the bottle was shaken in a reciprocating shaker (20.0 ± 0.5 °C; 150 rpm) for three days (Koh and Dixon, 2001). Because chloride anions may affect modification of zeolites and may change its characteristics (Prikryl and Pabalan, 1999), after filtration, the zeolite was rinsed several times with deionized water to remove residual chloride ions. In order to be sure about the absence of chloride ions in the modified samples, chloride anion was controlled by AgNO3 using Argentometry method in the supernatant samples (Ghiaci et al., 2004). At last, the samples were dried in ambient air for 48 h and stored in a desiccator. Modification of zeolites Zeolites can be modified by adsorption of surfactants on the zeolites surface. Formation of a monolayer of surfactants at the solid–aqueous interface via strong bonds is the most probable models of surfactants adsorption on a solid surface when the surfactant concentration in the solution is equal or less than its critical micelle concentration (CMC). If the surfactant concentration in solution exceeds the CMC, the surfactant molecules will form a bilayer of surfactants on the zeolite surface (Haggerty and Bowman, 1994). SMZS afford a hydrophobic environment for the partitioning of organic molecules with low polarity and high molecular weight (Wang and Peng, 2010). In this study, two types of surfactants, HDTMA-Cl (8; 14087; 0100*S4283687-532* HDTMA-Cl as an aqueous solution of 50%) and CPB (8; 18188; 0005* S4385288-625* N-CPB monohydrated) were used for modification of zeolitic-rich tuff. All chemicals used in this research were purchased from Merck Company. According to the references, the critical micelle concentration (CMC) of HDTMA and CPB is 1.8 mmol/L (Ghiaci et al., 2004). In order to determine the effect of surfactant concentration on the adsorption of MTBE, the zeolite was modified using HDTMA-Cl and CPB solutions with three initial concentrations: 0.5 ( MTBE adsorption The ability of raw or Non-Modified Zeolite (NMZ) and Surfactant-Modified Zeolites for removal of MTBE from aqueous solutions was carried out in a 125 mL screw-top brown glass bottles with 100 mL solution containing 10 mg/L of MTBE. 0.5 g/L of each previously prepared adsorbents (i.e. SMZ#1- SMZ#6 and NMZ) was added to the solution and then the bottles were shaken in a reciprocating shaker (20.0 ± 0.5 °C; 150 rpm; pH=7) for 24 h (the time that can be adequate to reach adsorption equilibrium (Torabian et al., 2010)). Then, the zeolite mixture was filtered and the filtered liquid was analyzed for MTBE concentration using a Varian Gas Chromatograph with a flame ionization detector and Combipal Headspace auto-sampler system (CP-3800, Varian, Australia). All adsorption tests were doubled run for the measurements of the MTBE concentrations and the averages are reported. Blank samples were also carried out using the same condition in the absence of adsorbent. Blank samples showed no significant change. The adsorption capacity and removal efficiency of adsorbed MTBE were determined as below:
Where: q is the MTBE adsorption capacity (mg MTBE/g adsorbent), Cin and Ct are the initial and residual concentrations of MTBE, respectively (mg/L), V is the solution volume (L), W is the dry weight of the adsorbent (g) and E is MTBE removal efficiency (%). At this step, the adsorbent with higher adsorption toward MTBE was selected for using in continuous experiments. To evaluate the effect of initial concentration and contact time on the adsorption of MTBE by SMZ, a fixed SMZ#3 dosage (i.e. 0.5 g/L) was added to 125 mL glass bottles with 100 mL solution of different concentrations of MTBE (i.e. 1, 10, 50, 100 and 200 mg/L) and stirred for varied contact times from 2 to 36 h (20.0 ± 0.5 °C; 150 rpm; and pH=7). Since pH varies depending on the nature of the water source and wastewater streams from different industries, pH can affect the adsorption process. Therefore, in order to evaluate the effect of the solution pH on the MTBE adsorption, experiments were conducted at different pH ranging from 4.0 to 10.0, while the examination conditions were the same as previous stage (excluding pH). The solution pH was adjusted using 0.1M hydrochloric acid and 0.1M sodium hydroxide solution before adding adsorbent to the vial. To control the solution pH, a Metrohm pH meter (827 pH lab, Switzerland) was used. In batch adsorption technique, the amount of adsorbent in the solution plays an important role. Therefore, the effect of the concentration of adsorbent on the adsorption of MTBE onto the selected adsorbent was examined using fixed concentrations of MTBE (10 mg/L) and varied concentrations of SMZ#3 from 0.2- 2.5 mg/L (20.0 ± 0.5 °C; 150 rpm; and pH=7). Isotherm studies The Langmuir and Freundlich isotherm constants in the linear equations can be calculated from the slope and intercept of the plot between Ce/qe vs. Ce, and log (qe) vs. log (Ce) respectively. On the other hand, an important characteristic of Langmuir isotherm can be obtained by a dimensionless factor called equilibrium factor (RL). Equilibrium factor can be defined according to the following equation: 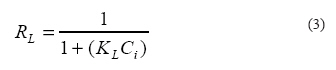
Where: Ci is the initial concentration of adsorbate (mg/L) and KL is the Langmuir constant (L/mg). If RL is in the range between zero and one, the adsorption condition is favorable (Acemioglu, 2004). Kinetic studies Batch experiments were carried out to analyze the rate constant of MTBE adsorption. The data obtained from kinetic adsorption, should be analyzed to understand the dynamics of the adsorption process and on the other hand, kinetic studies are useful for the prediction of adsorption reaction rate, modelling and designing the processes. Therefore, the most frequently used kinetic method like pseudo first-order; pseudo second-order and Elovich were used to represent the MTBE adsorption process. The pseudo first-order; pseudo second-order and Elovich Kinetic constants in the linear equation can be calculated from the slope and intercept of the plot between log (qe −qt) vs. t, t/qt vs. t and qt vs. ln (t), respectively. RESULTS The chemical analysis of the sample is shown in Table 2. The X-ray diffraction pattern was also obtained and its results are presented in Fig.1; the SEM image of the Miyaneh zeolite sample is illustrated in Fig. 2. For the zeolite used in this study (Miyaneh clinoptilolite), CEC and ECEC values were 2.38 Meq/g, and 0.648 Meq/g, respectively. According to the experimental results (Fig. 3), it is obvious that SMZ#3 is the most effective adsorbent; therefore, SMZ#3 was selected among seven other adsorbents mentioned above as the best adsorbent with highest MTBE adsorption capacity to be used in remaining experiments.The results of MTBE adsorption on SMZ#3 with different initial concentrations of MTBE (i.e. 1, 10, 50, 100 and 200 mg/L) and contact times (2- 36 h) are presented in Fig. 4. The trend of MTBE adsorption on SMZ#3 with different pH (i.e. 4 to 10) and with fixed initial concentration of 10 mg/L is presented in Fig. 5. The effect of SMZ#3 dosage on the sorption of MTBE was studied and the results obtained from this part of the study are illustrated in Fig. 6. Langmuir and Freundlich isotherms, which are given in Table 3, were used for the evaluation of the experimental results and the results of calculated isotherm models are listed in Table 4. Pseudo first-order, pseudo second-order and Elovich kinetics, given in Table 5, were used to represent the MTBE adsorption process and the obtained results from these three kinetic models are presented in Table 6. DISCUSSION Structural analysis The results showed relatively high amount of calcium compared to potassium and sodium, and the investigated Miyaneh natural zeolite was clinoptilolite and belonged to the Heulandite group. From Fig. 1, which shows the comparison between Miyaneh natural zeolite and reference clinoptilolite, it can be concluded that Miyaneh natural zeolite is mainly composed of clinoptilolite. Selection of the adsorbent According to the results (Fig. 3), SMZ#3 (i.e. sample that was modified with HDTMA with surfactant concentrations higher than the CMC) with nearly 80% adsorption was the most effective adsorbent, followed by SMZ#3, SMZ#6. Although, SMZ#3 was better than SMZ#6 in adsorption of MTBE, but the pattern of adsorption for both surfactants (i.e. HDTMA and CPB) were similar. Results are in agreement with the past researches, meaning that with respect to their nearly close CMC, scientists expect the HDTMA and CPB configurations on the surface of the sorbent to be similar (Ghiaci et al., 2004). As expected, Non-Modified Zeolite (NMZ) with nearly 9% adsorption is the worst adsorbent for MTBE removal. Natural zeolites have almost no affinity for anions or non-polar organics. By treating the zeolite with a cationic surfactant (for example HDTMA), an organic coating is created on the external zeolite surfaces and the charge is reversed. Anions are retained on the SMZ via anion exchange, while non-polar organic molecules partition into the surfactant coating (Karapanagioti et al., 2005). Non-polar organics such as the gasoline components benzene, toluene, and p-xylene are not adsorbed by raw zeolite, but in contrast, these compounds are strongly retained by SMZs (Bowman, 2003). Effect of initial concentration and time on the adsorption of MTBE Results from Fig. 4 show that in the range of MTBE concentration studied, the removal efficiency and adsorption capacity of MTBE by SMZ#3 increased rapidly in the first 10 h. The rapid uptake then gave way to a much slower adsorption after 10 h. This first rapid adsorption can be related to the concentration gradient that was created between MTBE concentration in solution and the SMZ#3 surface. At this rapid adsorption period, the maximum adsorption capacity of MTBE reached 91.6 mg/g and the maximum removal efficiency of MTBE to 83%, after which (i.e. more than 10 h) no significant change was observed. Thus, taking into account the results obtained in these experiments, 10 h was selected as the contact time for the remaining experiments. The experimental results (Fig. 4) showed that the removal of MTBE was highly concentration dependent. In different concentrations of MTBE, removal efficiency decreased with increasing MTBE concentration. Maximum removal efficiency took place at lower concentrations of MTBE (Fig. 4(a)) because; at lower initial concentration sufficient adsorption sites are available for sorption of MTBE molecules, therefore in lower concentrations the availability of free adsorption sites can overcome the MTBE initial concentration. Conversely the molecules of MTBE at higher concentration solution play as a driving force to overcome the mass transfer resistance between the aqueous and solid phases. Therefore, the adsorption capacity of SMZ#3 increased with the initial concentration of MTBE in solution as shown in Fig. 4(b). Effect of pH on MTBE adsorption Because, the weak positive charge of non polar compounds (such as MTBE) is maximum at lower pH, and the surface charge can be decreased rapidly by increasing the pH (Lu et al., 2008), it can be observed that maximum adsorption of MTBE by SMZ#3 took place at acidic range and decreased as a straight line with increase of the solution pH at the range from 4 to 8 and then droped rapidly after pH=8 (Fig. 5). The decrease in MTBE adsorption with pH increase is in agreement with the results of a study on adsorption of BTEX using surfactant-modified natural zeolite (Torabian et al., 2010). Authors concluded that the removal efficiency of BTEX decreased as a straight line from 95% to 85% with increasing the pH from 3.0 to 10.0 and then droped rapidly after pH=10. They reported that surfactants specially HDTMA, are stable at various pH ranges with high or low ionic strength. Therefore, possibility of degradation of SMZs or desorption of surfactant from the surface of clinoptilolite is very low. Effect of adsorbent dosage The amount of MTBE adsorbed per gram of the SMZ#3 (mg/g) reduced with increasing the dosage of SMZ#3, but the MTBE removal efficincy increased (Fig. 6). This shows that the equilibrium adsorption capacity and removal efficiency of MTBE depends on of the adsorbent dosages. Increasing the dosage of the SMZ#3 and keeping the MTBE concentration constant makes a large number of free sites available for a fixed concentration of MTBE that cause higher removal efficiency. On the other hand, with increasing the dosage of the adsorbent and a constant concentration of MTBE, the driving force of MTBE molecules were decreased and finally lower adsorption capacity was resulted. Isotherm studies As shown in Table 5, for three adsorbent doses (0.5, 1.5, 2.5 g/L), Langmuir isotherm was the best fitted for describing the results. It has been found that SMZ#3 with concentration of 0.5 g/L shows the maximum adsorption capacity (qm) of 100 mg/g. The RL values obtained from this study were 0.34 to 0.5, which indicate favorable adsorption of MTBE by SMZ#3 (Acemioglu, 2004). Kinetic studies Pseudo second-order gave better kinetic results (i.e. correlation coefficients were closer to 1) than other two kinetic models at varied initial concentrations of MTBE. The R2 values of pseudo second-order fluctuated from 0.979 to 0.995 and the adsorption constant rate by SMZ#3 was obtained as 0.245 (g/mg.min) at 1 mg/L of MTBE. Since surfactant-modified natural zeolites are capable of adsorption and removal of organic materials from aqueous solutions, they could be a good candidate for removal of natural organic matters detected in low concentration levels in raw surface water sources of Tehran (Zazouli et al., 2007). ACKNOWLEDGEMENTS The authors highly appreciated the financial support of this research by Deputy of Research, Tehran University of Medical Sciences. The authors also wish to acknowledge the Pars Oil and Gas Company for financial support of this study. REFERENCES
Copyright 2010 - Iran Journal of EnvironHealth Sci Eng The following images related to this document are available:Photo images[se10027t4.jpg] [se10027t3.jpg] [se10027f3.jpg] [se10027f6.jpg] [se10027f1.jpg] [se10027t6.jpg] [se10027f4.jpg] [se10027f2.jpg] [se10027t5.jpg] [se10027t1.jpg] [se10027t2.jpg] [se10027f5.jpg] |
| |||||||||