 |
| About Bioline | All Journals | Testimonials | Membership | News |
|
||||||
|
||||||
Iranian Journal of Environmental Health, Science and Engineering, Vol. 8, No. 2, 2011, pp. 101-108 Equilibrium And Kinetics Study Of Reactive Red 123 Dye Removal From Aqueous Solution By Adsorption On Eggshell 1M. H. Ehrampoush, 2Gh. Ghanizadeh, *1 M. T. Ghaneian 1Department of Environmental Health, Faculty of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Received 13 June 2010; revised 23 September 2010; accepted 17 January 2011 Code Number: se11012 ABSTRACT The aim of this study was to determine the equilibrium and kinetics adsorption of reactive red 123 dye (RR 123) from aqueous solution with chicken’s eggshell as an adsorbent. The initial concentrations of reactive red 123 dye were selected in the range of 25 and 50 mg/L. The target adsorbent was prepared in laboratory conditions and pulverized by ASTM standard sieves. Measurement of the adsorbent surface area was carried out via Brunauer-Emmett-Teller isotherm. The experimental data were analyzed with Langmuir, Freundlich and Temkin isotherm models. The results showed that the calcium components were the main constituents of eggshell (around 95% Ca). The experimental adsorption isotherm was in good concordance with Langmuir and Freundlich models (R2>0.90) and based on the Langmuir isotherm the maximum amount of adsorption (qmax) was 1.26 mg/g. Increase of the eggshell dose and the solution temperature beyond 45ºC led to decrease of the adsorbed dye per mass unit of the adsorbent, but increase of the solution pH up to 9 led to the improvement of dye adsorption. The kinetic studies revealed that the adsorption of reactive red 123 was rapid and complied with pseudo-second order kinetic (R2= 0.99), with the kinetic constant of 0.02 g/mg.min. Key words: Eggshell waste; Reactive red 123 dye; Adsorption; Sorption kinetics; Natural sorbent INTRODUCTION Synthetic dyes are extensively used in the textile industry. Due to inefficiencies of the industrial dyeing process, some of the used dyes are lost in the effluents of textile units, rendering them highly colored (Boer et al., 2004; Maleki et al., 2010). Direct discharge of these effluents causes formation of toxic materials in receiving media. In addition to their visual effects and their adverse impacts in terms of chemical oxygen demand, many synthetic dyes are toxic, mutagenic and carcinogenic (Rezaee et al., 2008; Dehghani et al., 2008). Degradation of dyes, especially the reactive dyes is difficult, due to their complex structure, water solubility and synthetic nature. Therefore, it is necessary to find the effective methods of wastewater treatment capable of removing color and toxic organic compounds from textile effluents (Netpradita et al., 2004; Mahvi et al., 2009). Current studies show that adsorption is one of the most promising techniques for quick lowering of the concentration of dissolved dyes from aqueous solutions (Aksu, 2005). In this regard, activated carbon has been evaluated extensively for the removal of color resulting from the different classes of dyes and is now the most widely used adsorbent for dyes. The application of carbon-based adsorbent still remains an expensive process due to the high cost in the using of activated carbon and difficulties in regeneration of the spent activated carbon. Hence, there is growing interest in utilizing biomass wastes/alternatives to activated carbon as low-cost adsorbents (Tsai et al., 2008 a). Based on the bioresource recovery and reuse, the high value of the utilization of eggshell (ES) waste known as a food processing by-product, has slightly increased in recent years. The use of chicken eggshell has been introduced as a possible bone substitute, as the starting material for preparing calcium phosphate bioceramics (e.g., hydroxyapatite), and as a low-cost adsorbent for removal of ionic pollutants from aqueous solutions (Tsai et al., 2008 b). The objective of this research was to evaluate the feasibility of eggshell for reactive red 123 dye removal from aqueous solution and determination of adsorption equilibrium and kinetics. MATERIALS AND METHODS Reagents and analytical procedure Reactive red 123 dye (RR123) was obtained commercially from Dye Star Company and used as an adsorbate without purification. It was dried at 110°C for 2 h before use (Sariglu and Aatay, 2006). Dye aqueous solutions were prepared with tap water. All other chemicals were guaranteed or analytic grade reagents commercially available and used without further purification. Because the maximum absorbance wavelength (λmax) of RR 123 was not reported in other studies, this parameter was determined with absorbance spectrum detection between 200 nm to 800 nm using the UV-visible spectrophotometer (PU 8700). The absorbance spectrum curve showed that the λmax of RR 123 was 506 nm. The concentration of dye was measured at this λmax with the calibration curve which was prepared by the use of the standard solutions of RR 123. Preparation of eggshell Granular eggshell was prepared in the laboratory conditions (20-25 oC). The eggshell obtained from commercial confectionaries were crushed into pieces of 10-15 mm in length , rinsed three times with distillated water and pulverized by standard ASTM sieves with the range of 8.00 to 14.00 mesh (2.83-1.41 mm) (Purevsuren et al., 2004; Choy and McKay., 2005 ; ASTM, 2007). The effective size (D10), D60 and uniformity coefficient (UC) of the adsorbent were 3.00 mm, 5.10 mm and 1.70, respectively. The prepared eggshell was dried at 100-110 °C over night, cooled in a desiccator and used as an adsorbent in the experiments (Rezaee et al., 2009). Characterization of eggshell The chemical composition and solid structure of ES was analyzed using scanning electronic microscopy and energy dispersive analysis X-ray (EDAX) spectra (Model XL30 Philips). Other surface specifics of ES were characterized by the Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) isotherms (Orfão et al., 2006; Ghanizadeh et al., 2010 ). The BET and BJH specific surface areas were determined by nitrogen adsorption-desorption measurement and applying of the relevant equations within P/P0 0-1 (P and P0 represent the operational and absolute pressures, respectively) (Vágvölgyi et al., 2008). Also, the porosity of ES was determined with the conventional adsorption of N2 gas at 77 °K and pressure of 91.43 KP (Shieldes et al., 2004). Analysis of data for calculation of the BET and BJH surface areas (m2/g), pore volume and porosity was carried out by Belsorb software (Ver.5) (data not shown). Adsorption experiments All the adsorption experiments were carried out by batch technique. The kinetic adsorption studies were performed at different doses of adsorbent, pH and dye concentrations. The initial concentrations of RR 123 dye in the experiments were selected in the range of 25 to 50 mg/L. The dye solution (50 mL) of desired concentration at neutral pH was taken in Al-covered glass vials and agitated with a known weight of ES at room temperature (25±0.5 °C) in a shaker set at 150 rpm (GFL 3017) for a desired time periods. The preliminary kinetic experiments revealed that 120 min was long enough to achieve the equilibrium. Therefore, a contact time of 2 h was selected for the entire equilibrium test. The solution pH was carefully adjusted by adding a small amount of HCl and NaOH solutions (0.1N), which measured using a pH meter (Mettler 8603). At the end of adsorption tests, the suspensions were centrifuged for 5 min at 2000×g and the concentrations of residual dye were determined using Philips UV/ visible PU 8700 spectrophotometer corresponding to λmax of RR 123 (506 nm). Blank solution with no dye was used for each series of experiments. Dilutions were carried out when measurement exceeded the linearity of the calibration curve. The amounts of adsorbed dye at equilibrium and dye removal efficiency were calculated from the mass balance equation 1 and equation 2, respectively, as follows:
where: Ce and Co are the equilibrium and initial concentrations of dye (mg/L), respectively; qe is the equilibrium dye concentration on adsorbent (mg/g); V is the volume of dye solution (L); M is the mass of adsorbent (g) and E is the removal efficiency (Karaoğlu et al., 2009 ; Ghanizadeh et al., 2010). The effect of adsorbent dosage was studied by changing the amount of ES mass from 1 to 5 g. Adsorption characteristics In adsorption systems the accurate determination of the maximum adsorption capacity used for process design is important. The Freundlich, Langmuir and Temkin models were used to describe the equilibrium between the adsorbate and the adsorbent, which can be represented respectively as follows (Farah et al., 2007):
k and n are constants that depict the adsorption capacity and intensity, respectively. The linear form of the Langmuir isotherm equation can be described as:
qmax is the maximum amount of adsorption(mg/g) and b is the adsorption equilibrium constant(L/mg). Temkin isotherm is represented by the following equation:
The linear form of this equation can be expressed as:
where: The experimental data were analyzed according to linear form of isotherm equations (Eqs 3, 4, and 6). Analysis of data for selection of the best fit isotherm as carried out by linear regression analyses of these models and comparison of the correlation coefficients (R2) (Farah et al., 2007). In order to describe the adsorption process type and the adsorption affinities between the adsorbent and adsorbate, the dimensionless separation factor RL was calculated from the following formula Kinetic parameters and energy of adsorption The kinetic of RR123 dye adsorption on ES was evaluated with the pseudo-first order and pseudo-secondorder models. For the analysis of data with the first model, the linear form of Lagergren equation was used, which its integrated form can be expressed as: 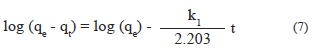
where qt is the amount of dye adsorbed per unit weight of adsorbent at time t (mg/g) and k1 is the rate constant for pseudo-first order kinetic which can be calculated from the plot of log (qe-qt) versus time. Since this model may not fully describe the adsorption kinetics, we used the pseudo-second-order equation. This model is often successfully used to describe the kinetics of fixation reaction of adsorbate on adsorbent surface. The linear and integrated form of this model can be described as:
where k2 is the rate constant of adsorption. The equilibrium adsorption capacity (qe) and the rate constant k2 (g/mg.min) were determined with plot of (t/ qe) versus time (Yu et al., 2009). RESULTS Adsorbent characteristics The results of energy dispersive X-ray (EDX) spectra analysis demonstrate that calcium is the main component of ES (Fig. 1). As shown in Table 1, analysis of ES component with EDX showed that calcium and phosphorous as CaCO3 and Ca3(PO4)2, respectively, were the dominant elements in ES structure. These results represent that the ES can be categorized as a homogeneous adsorbent which contains around 95% Ca and has only 4% organic mater. Analysis of surface characteristics with BET and BJH models showed that the specific surface area of ES was 1.2 m2/g and the sizes of the pores were lower than 20 nm. Also, BJH analysis showed that the pores of this adsorbent did not have uniform sizes (data not shown). Effect of adsorbent dose Variation of adsorbent dose showed that although increasing of ES dose in aqueous solution can result to increased pollutant removal, but this elevation of ES leads to decreasing of adsorbed dye per unit of adsorbent (qe); this phenomenon may be related to the use of surface area as unsaturated form. The results of this research showed that with increasing of ES from 0.5 g to 5 g, the qe (mg/g) decreased from 0.82 mg/g to 0.4 mg/g (Fig. 2). This result indicates that although mass elevation of adsorbent can provide large or available surface area, but the adsorption pattern of the pollutant as unsaturated form leads to unfavorable using of adsorbent. This phenomenon is the most important point for the design of economical and large scale adsorption devices. Effects of dye concentration and contact time The effects of contact time and initial concentration of dye on RR123 adsorption at room temperature are shown in Fig 3. The quantity of adsorbed solute (mg/g) increased with lapse of contact time and the equilibrium reached after 120 min. This figure shows the adsorption or migration rate of dye molecules from liquid to solid phase. The qe for C0= 25 and 50 mg/L were 0.45 mg/g and 0.62 mg/g, respectively. These results implies that the removal of RR 123 with ES depends on dye concentration and may be related to driving forces that need to overcome for the resistances of pollutants migration from the aqueous solutions to the ES surface. Effects of pH and temperature The relevant surface properties and predominant constituents of ES are presented in Table 1. The pH of aqueous solutions is a key factor on adsorption process which is a function of hydrogen and hydroxyl ions concentrations. The effects of solution pH on RR 123 adsorption within ES were investigated and the results are illustrated in Fig. 4, which shows that the elevation of pH leads to increasing of the adsorbed mass of pollutant (qe) onto ES. When the pH of aqueous solution increased from 5 to 9, qe increased from 0.3 to 0.5 mg/g. Based on this phenomena it can be concluded that elevation of the aqueous pH to higher scale may lead to increasing of ionisable charge sites and adsorbed dye on the ES surface. Temperature of the adsorption process is another factor that can influence the efficacy of these systems. In this research the effect of temperature on RR 123 adsorption within ES was studied (Fig. 5). This figure shows that the fluctuation of temperature in studied range has different effects on RR 123 adsorption with ES. As shown in Fig. 5, increasing of temperature from 15 to 25º C leads to elevation of adsorbed dye from 2.61 mg/g to 2.78 mg/g , but temperature increase from 25ºC to 55ºC causes the decreasing of qe to 1.49 mg/g. These results show that, although the enhancing effects of temperature on RR 123 adsorption is not important, but inhibition effects of the higher temperatures are significant and should be considered as an operational parameter in economical equipments. Sorption experiments/Adsorption isotherm Application of Langmuir, Freundlich and Temkin models to the adsorption isotherm, showed that the Freundlich and Langmuir isotherm models provided excellent satisfactory with the highest R2 value (0.93 and 0.91) compared to the Temkin model. The estimated values for the parameters of these models are demonstrated in Table 2. Although the correlation coefficient of the Freundlich isotherm is slightly higher than the Langmuir isotherm, but the experimental studies with other operational parameters such as pH and temperature showed that the calculated qe (mg/g) in various pH and temperature complies with qe that was determined with Langmuir isotherm; so, it can be derived that the Langmuir isotherm gave better fits than the other isotherms in which the maximum uptake capacity for RR 123 was 1.26 mg/g. Based on dimensionless separation factor (RL) on Langmuir model the value of this parameter for RR 123 adsorption within ES is lower than 1 which confirms that adsorption of this dye with this material is favorable under the conditions of this research. The results of the kinetics of adsorption process are shown Table 3, Analysis of data and regression coefficient (R2) represent that the adsorption of this dye complies with pseudo- second order kinetic. Thus the correlation coefficient of this model is higher than 0.99 and the calculated adsorption capacity complies with experimental values. The values of k1 and k2 were calculated as 0.041 and 0.063 g/mg.min for pseudo- first order and pseudo-second order kinetics, respectively. DISCUSSION As shown in Table 1 and Fig. 1, the dominant constituents of ES are Ca and P. Similar results were reported by Tsai et al (2006). Table 1 shows the BET surface area and the pore volume of ES were determined to be 1.2 m2/g and 0.0062 cm3/g, respectively. These results are confirmed by Tsai et al., (2006). Fig. 2, shows that although increasing of the ES dose led to increasing of dye removal efficiency but resulted in decreasing of adsorbed dye per mass unit of ES. This result may be related to occupation patterns of active sites and may be in higher doses of ES, the active sites of adsorbent occupied as unsaturated form that may be related to particles aggregation and overlapping interfere with binding sites which reduces the total surface area and adsorption quantity. (Yeddou and Bensmaili, 2007; Mezenner and Bonsmaili, 2009). Fig.3, shows in the same contact time, increasing of RR123 dye concentration led to increasing of qe(mg/g). This phenomenon may be related to the driving forces that need to overcome for the resistances of pollutants migration from the aqueous solutions to the ES surface (Hameed and Ahmad, 2009). Also, Fig. 3 represents that vacant sites on Es surface has the key role on pollutant adsorption so, in preliminary steps of contact time, the adsorption rate of dye is higher and the lapse time leads to reducing of this rate, because in progressed contact time, the remaining vacant sites are difficult to be occupied due to repulsive forces between the molecules of the ES surface and the aqueous solution (Mezenner and Bonsmaili , 2009). As shown in Fig. 3 the adsorption of RR123 onto ES reached to equilibrium after 120 min that complies with Tsai et al., (2008 b) results. (Tsai et al., 2008 b). Fig. 4 shows the effect of pH on RR 123 dye removal within ES. This result may be related to ionization degree of dye molecules and relevance functional groups of ES surface (Zheng et al., 2007). Also, this phenomenon may be related to electrostatic interactions which appear between positive and negative charges of RR 123 species and adsorbent surface (Bilgiç., 2005 ; Medelline-castillo et al., 2007 ). Fig.5 that shows the elevation of the solution temperature from 15°C to 45°C led to increasing of qe from 0.3 mg/g to 0.6 mg/g. This phenomenon revealed that elevation of solution temperature increases the dye molecules mobility and number of molecules that acquire sufficient energy to undergo an interaction with ES surface (Hameed et al., 2007). This observation was similar to other researches reported (Tsai et al., 2008 b). Furthermore increasing of temperature may lead to swelling effect in internal surface of the ES enabling large quantities of RR 123 dye to penetrate further onto ES structure. Table 2 revealed that the adsorption of RR123 dye onto ES can be described with the Langmuir and Freundlich isotherms. These findings are in accordance with Zhang et al.,(2007) results. The similarity of these results may be related to adsorbent characteristics especially in term of chemical constituents. Tsai et al., (2008 b) reported that the adsorption of Methylene blue within ES was well fitted with Freundlich isotherm model. Although the findings of this experimental study is not in accordance with Tsai et al.,(2008 b) results but survey of the correlation coefficients of adsorption models which were reported by these researchers show that the differences between these coefficients are not significant and can be ignored (R2=0.9 for Langmuir model vs. 0.94 for Freundlich model). Table 3 represents that the adsorption of RR 123 onto ES was well fitted with pseudo-second-order kinetic (R2= 0.99). Tsai et al.,(2008 b) studied the adsorption kinetic of cationic basic blue, and anionic acidic orange 51 onto calcified eggshell and ground eggshell powder and reported that the adsorption of these dyes in these adsorbents complies with pseudo-second order kinetics (Tsai et al., 2008 b). Based on these findings it is shown that although the adsorbate specificities have a key role in adsorption kinetic characteristics, but in adsorption studies that ES was used as an adsorbent; the adsorbent identification has a predominant role. ACKNOWLEDGEMENTS Authors are grateful of Chairman of Environmental Health Department, Shahid Sadoughi University of Medical Sciences for providing instruments and facilities of this research and Prof. Naghii for his scientific comments and efforts in preparing of this article. REFERENCES
Copyright © 2011 - Tehran University of Medical Sciences Publications The following images related to this document are available:Photo images[se11012t1.jpg] [se11012f1.jpg] [se11012t2.jpg] [se11012f4.jpg] [se11012e8.jpg] [se11012t3.jpg] [se11012f3.jpg] [se11012f5.jpg] [se11012f2.jpg] |
| |||||||||