 |
| About Bioline | All Journals | Testimonials | Membership | News |
|
||||||
|
||||||
International Journal of Enviornmental Science and Technology, Vol. 4, No. 4, Autumn 2007, pp.525-532 Potentiality of open burnt clay as an adsorbent for the removal of Congo red from aqueous solution *M. A. Mumin; M. M. R. Khan; K. F. Akhter; M. J. Uddin Department of Chemical Engineering and Polymer Science, Shah Jalal University of Science and Technology,Sylhet-3114, Bangladesh Received 28 February 2007; revised 28 March 2007; accepted 1 August 2007; available online 1 September 2007 Code Number :st07068 ABSTRACT Open burnt clay was studied as a potential adsorbent for the adsorption of Congo red (a reactive dye) from aqueous solution. The effect of contact time, pH, adsorbent dosage and temperature were studied. It was observed that the amount of Congo red retained increase with decreasing pH and increasing initial concentration. Removal percentage at pH 2 and 3 are almost same. The adsorption capacity of regenerated burnt clay was showed more than 98 % recovery of the adsorption efficiency of initial virgin adsorbent. The equilibrium data were described well by both Langmuir and Freundlich isotherm model. The adsorption capacity of some natural adsorbents, namely rice husk, wood charcoal, tea waste etc. were also investigated and compared with that of open burnt clay. Key words: Adsorption, wastewater, open burnt clay, Congo red INTRODUCTIONOne of the major problems concerning textile wastewater is colored effluent. The discharge of color waste is not only damaging the aesthetic nature of receiving streams but also it may be toxic to aquatic life. In addition, color interferes with the transmission of sunlight into the stream and therefore reduces photosynthetic action. According limits of color concentration in effluent are permissible (Metcalf and Eddy, 1991). The color in the effluent is mainly due to unfixed dye. The concentration of unused dyes in the effluent depends upon the nature of dyes and dyeing process underway atthetime(McMullan, et al., 2001). Inefficiency of dyeing process results in 10-25 % of all dye stuffs being lost directly to the wastewater (Perineau, et al., 1982). Although the textile dyes contribute only a small portion of the total volume of discharged wastewater after the dyeing process, yet they make it deeply colored (McKay, et al., 1985). The removal of dye in an economic fashion remains an important problem. Considerable work has been carried out on the removal of dye from wastewater (Perineau, et al., 1982; McKay, et al., 1985; Gupta, 1985; Khattri, 2000; Low, et al., 2000; Liversidge, et al., 1997; Choy, et al., 1999, Asilian, et al., 2006). Water insoluble dyes (e.g. disperse and vat dyes) generally exhibit good exhaustion properties i.e. most of the dye bonds to the fibre and have been reported to be removed by physical means such as flocculation. When effluents containing these classes of dyes are discharged to a conventional sewage treatment works most of the color is removed by adsorption on biomass. However since the introduction of water soluble dyes (reactive dyes), which are extensively used in the industry, conventional biological treatment processes are no longer able to achieve adequate color removal. Adsorption appears to offer the best prospects over all the other techniques (Robinson, et al., 2001; Kamel, et al., 1991; Keith, et al., 1999; McKay, 1981). The most widely used physical method for dye removal is activated carbon (Nasser and El-Geundi, 1991, Molla and Robinson, 1996). Synthetic clays showed a high adsorption for reactive dyes, furthermore, the adsorption capacity for this adsorbent exceeded or almost same that of activated carbon (Lambert, et al., 1997, Sethuraman and Raymahashay, 1985, Singh, et al., 1984). A number of biological adsorbents have also been investigated for the removal of reactive dyes, these includeamongst others; applepomaceandwheat straw (Robinson, et al.,2002), corncoband barley husk (Robinson, et al., 2001), maize cob, wood and rice hull (Low and Lee, 1997). These adsorbents were found to be efficient in binding with basic dyes rather than acid dyes. In this work the adsorption capacity for reactive dye Congo red was determined using open burnt clay. Open burnt clay was selected because of its high adsorption capacity for a large number of contaminants in aqueous solution. The parameters that influence adsorption such as dye initial concentration, contact time, solution pH and adsorbent dose were investigated. Adsorption capacity of regenerated open burnt clay was compared with that of fresh adsorbent. The removal of the dye from aqueous solution by some natural adsorbents such as tea waste, wood charcoal, rice husk etc was also studied. This research work has been done at the Research Laboratories, Department of Chemical Engineering and Polymer Science at the Shah Jalal University of Science and Technology, Sylhet-3114, Bangladesh during February-July 2005. MATERIALS AND METHODSSoil (24.76 % Fe2O3, 5.75 % Al2O3, 58.29 % SiO2) (Sadi and Alam, 1997) collected from the hilly sites of Shah Jalal University of Science and Technology, Sylhet, Bangladesh, was washed with distilled water three times to free it from pebbles and other unexpected particles. The residue was then dried at 105 ºC for an hour. A desired amount of dry clay was placed in a crucible and burned with the help of a gas burner for fifteen minutes which was used as adsorbent. The particle size of adsorbent was 100-400 mesh and bulk density was measured as 1.2 g/cm3.The reactive dye Congo red (MERCK, Germany) was used as adsorbate. Stock solution of Congo red was made using double distilled water. All working solutions were prepared by diluting the stock solution with double distilled water. The pH adjustments were made with HCl or NaOH solutions. The concentrations of dye were determined spectrophotometrically (UV-spectrophoto-meter, Model: UV-1601, Shimadzu, Japan) by monitoring the absorbance at 498 nm for neutral pH and at 558 nm for pH in acidic range, where maximum absorbances were observed. Batch sorption tests were done at 26±2 °C temperature using a flash shaker at 350 rpm in 250 mL conical flasks. In the adsorption isotherm tests, 1.5 g adsorbent was thoroughly mixed into 200 mL dye solution in the range 40-100 mg/L. The initial pH was adjusted at 3 by HCl solution. After the flasks had been shaken for 7 h., the solution was centrifuged for the separation of solid particles before spectrophotometric measurements of dye. Adsorption kinetic tests were done for the initial concentration range 40-100 mg/L, adsorbent amount 1.5 g at pH=3. For pH studies, 1.5 g adsorbent was thoroughlymixed into 200 mL dye solution having initial concentration of 50 mg/L. The solution was agitated for 7 h. Shaking time for all cases was 7 h. because at that time system reached to the equilibrium RESULTS The analysis of isotherm data is useful for design purpose. In present study the equilibrium data were treated by Langmuir and Freundlich isotherms. The Langmuir isotherm can be represented by the following equation (McKay, 1981). 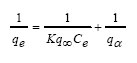 (1) (1)
where, qe is the amount adsorbed per unit mass of sorbent at equilibrium (kg/kg); qα is the maximum adsorption capacity (kg/kg); Ce is the equilibrium dye concentration (mg/L) and K is the adsorption equilibrium constant (m3/kg). The plot of 1/qe vs 1/Ce is linear which show that the adsorption of dye onto burnt clay follows Langmuir isotherm model (Fig. 1). qα and K were calculated from the slope and intercept of the plot and were found to be q ∞ =22.83×10-3 kg/ kg and K = 0.55×103 m 3/kg. The Freundlich isotherm was also applied for the adsorption of dye by burnt clay (McKay, 1981). log q e= (1 / n ) log C e + log k f (2) where, qe is the amount adsorbed per unit mass of adsorbent at equilibrium (kg/kg); Ce is the equilibrium dye concentration of the solution (mg/L). kf and n are the Freundlich constants, n gives an indication of the favorability and kf [kg/kg(m3/kg)n], the capacity of the adsorbent (Netpradita, et al., 2003). From Fig. 2, it can be seen that the equilibrium data can be fit satisfactorily with Freundlich. The value of kf and n were found to be 0.298 and 3.802 respectively. The value of n lies between 2 and 10, which implies good adsorption (Mc Kay, et al., 1985). Effect of contact time and initial dye concentration on dye removal was investigated using dye concentrations 40-80 mg/L and a pHin = 3. The results are presented in Fig. 3 and show that dye uptake by burnt clay reached equilibrium in approx. 200, 300, 350 and 420 min for dye concentrations of 40, 50, 60 and 80 mg/Lrespectively.The removal of dye was rapid in the initial stages of contact time and gradually decreased with time until equilibrium. The effect of pH on Congo red removal was studied at different pH values. The results are presented in Fig. 4. When pH of dye solution was increased form 2 to12, the percent removal decreased from 94 % to 2 %. Influence of adsorbent concentration on the amount of Congo red adsorbed by open burnt clay is shown in Fig. 5. It can be seen that by increasing the adsorbent dose from 0.5-3 gm (for initial dye concentration 50 mg/L, solution volume 200 mL andat a pH=3)the removal efficiency increases. The number of available adsorption sites increases by increasing the adsorbent dose and that results in the increase of removal efficiency. Clay is very much cheap so regeneration is not necessary. In our present study we did the regeneration of used burnt clay to check the adsorption capacity of regenerated burnt clay. The conventional method for regeneration of spent adsorbent is thermal treatment of the sorbed organics in a controlled atmosphere that minimizes the oxidation of the adsorbent particles itself. In our present study the used burnt clay is openly burnt to regenerate and reuse. The adsorption capacity of the regenerated burnt clay was found 0.02038 kg/kg, i.e. the capacity decreases by calculation is only 1.1 %. DISCUSSION AND CONCLUSION Adsorption of Congo red onto burnt clay followed both Langmuir and Freundlich isotherm. The adsorption capacity of burnt clay was 22.83Χ10-3 kg/ kg adsorbent. - The essential characteristics of Langmuir isotherm can be express by a dimensional constant called equilibrium parameter, RL (Nasser,1991)that is defined by,
where, K is the Langmuir constant at m3/kg and Co is the initial concentration. The value of RL indicates the shape of the isotherm to be either unfavourable (RL >1), linear (RL=1), favourable (0< RL<1) or irreversible (RL=0). As shown in Table 1, RL value decreases with the concentration and indicates favorable adsorption for dye on clay. In the Freundlich isotherm, the Freundlich constant n gives an indication of the favorability. The value of kf and n were found to be 0.298 and 3.802 respectively. The value of n lies between 2 and 10, which implies good adsorption (Mc Kay, et al., 1985). During Kinetic study it has been seen that the removal of dye was rapid in the initial stages of contact time andgradually decreased with timeuntil equilibrium. This decreasing removal rate towards the end, suggests formation of monolayer coverage of dye molecules on the outer surface of the adsorbent and pore diffusion onto the inner surface of the adsorbent particles through the film due to continuous agitation maintained during the experiments. The rate constant for the adsorption of dye on burnt clay was determined using Lagergren equation (Gueu et al., 2007, Islam et al. 2004). log (qe −q)=log qe − kadt / 2.303 (4) where, qe and q are the amounts of dye adsorbed (kg/ kg) at equilibrium and at timet (min), respectivelyand kad is the rate constant of adsorption. The kad values are determined by fitting the kinetic data to Eq.4 and presented Table 2. The rate constant is found to be changed with initial concentration (Fig. 7). It may be concluded from the data fitting in the rate expression that the reaction taking place is of the first order. Sorption of Congo red onto burnt clay is pH dependent and optimum pH was 3. The kinetic data reveals that the adsorption takes place follows the first order reaction kinetics. The adsorption capacity of regenerated burnt clay was 20.38Χ10-3 kg/kg, which is almost similar with that for initial virgin adsorbent. From the pH study it can be concluded that in acidic media the dye removal percentage is higher. The decrease in adsorption with pH may be explained on the basis of aquacomplex formation and subsequent acid-base dissociation at solid solution interface. Any oxide surface creates a charge (positive or negative) on its surface (Netpradita, et al., 2003). This charge is proportional to the pH of the solution that surrounds the oxide particle. The structure of Congo red and the change in color from red to blue in presence of mineral acid is due to the resonance hybrid of (1) and (2) structures given below (Rahman, et al., 1999). The structure of Congo red and the change in colour from red to blue in presence of mineral acid is due to the resonance hybrid (Rahman, et. al., 1999). Congo red contains an azo (-N=N-) chromophore and an acidic auxochrome (-SO3H) associated with the benzene structure. Congo red is also called acidic azo dye. Azo structure is more important because of its high ability to impart color to the compound. Structure (2) in acid media, posses two +NH2 groups, which shows affinity to attract negatively charged silica /silicate anion in clay. Lower pH, it is usual that clay shows a great adsorptive power to Congo red. It can also be seen that by increasing the adsorbent dose from 0.5-3 gm the removal efficiency increases. The number of available adsorption sites increases by increasing the adsorbent dose and that results in the increase of removal efficiency. Another reason may be due to the aggregation/agglomeration of adsorbent particles at higher concentration. Such aggregation would lead to decrease in total surface area of soil particles available to Congo red adsorption and an increase in diffusional path length (Al-Degs, et al., 2000). The particle interaction action brought about by high sorbet concentration may also desorb some of the adsorbate which is only loosely and reversibly bond to the carbon surface. The data can also be used to derive a mathematical relationship to relate the Congo red removal to the adsorbent dose. - This relationship, for which the correlation coefficient (R) was 0.913, is
This equation can be used to predict the percentage Congo red removal for any adsorbent dose with in the test limits to the experimental conditions. Theremoval efficiency was studied for an initial dye concentration of 50 mg/L and different adsorbent doses. Optimum results are shown in Fig. 6. It is evident that the congo red removal by activated charcoal approaches toalmost 100 %, but it is expansive, can not be disposed in the environment and regeneration is essential. Tea waste and base treated clay showed almost 40 % removal of Congo red, where as the open burnt clay showed comparable results with that of activated charcoal. The results obtained in this study clearly demonstrated the potentiality of burnt clay for the removal of Congo red from aqueous solution. It is also evident that activated charcoal is most superior adsorbent but it is expansive, can not be disposed in the environment and regeneration is essential. On theother hand, industrially or in caseof large scale operation regeneration of adsorbent by desorption is not feasible. Therefore burnt clay could be the only alternative of activated charcoal in this process. ACKNOWLEDGEMENTSAuthors are indebted to the Shah Jalal University of Science and Technology, Sylhet, Bangladesh for their support. REFERENCES
© 2007 Center for Environment and Energy Research and Studies (CEERS) The following images related to this document are available:Photo images[st07068f4.jpg] [st07068f6.jpg] [st07068f5.jpg] [st07068f2.jpg] [st07068f7.jpg] [st07068f1.jpg] [st07068f3.jpg] [st07068t1.jpg] [st07068t2.jpg] |
| |||||||||