
|
African Journal of Traditional, Complementary and Alternative Medicines
African Ethnomedicines Network
ISSN: 0189-6016
Vol. 5, Num. 4, 2008, pp. 355-362
|
African Journal of Traditional, Complimentary and Alternative Medicines, Vol. 5, No. 4, 2008, pg. 355 - 362
Research Paper
Antinociceptive
and antiinflammatory effects of essential oil of Dennettia tripetala g. Baker (Annonaceae) in rodents
I. A.
Oyemitan1*, E. O. Iwalewa 1, M. A. Akanmu,1 T.
A. Olugbade 2
1Department of Pharmacology, 2Pharmaceutical
Chemistry, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria. *Email: oyemix @yahoo.com
Code Number: tc08049
Abstract
In this study we evaluated the analgesic and anti- inflammatory
activities of the essential oil (EO) of the fruits of Dennettia tripetala
in rodents. The plant is a tropical African plant and the fruits are commonly
eaten as spices and consumed as a stimulant, and its various parts are used in
the treatment of fever, cough and as anti-emetics.The analgesic effects of the
oil was assessed in mice using the hot plate, acetic acid-induced writhings and
formalin test, while carrageenan-induced paw oedema was used to study the
antiinflammatory effects in rats.The EO at 25-50 mg/kg exhibited
significant (p<0.05) antinociceptive effects comparable to a potent opioid
agonist, morphine (10 mg/kg) and non-steroidal anti-inflammatory drugs such as,
aspirin (100 mg/kg) and indomethacin (80 mg/kg). The antinociceptive effect of
the EO was also blocked by naloxone (2 mg/kg) in all the models used. The EO
demonstrated significant (p<0.05) anti-inflammatory effect in the
carrageenan-induced paw oedema model of inflammation that is also comparable to
dexamethasone (1 mg/kg) The results showed that the essential oil of D.tripetala
possesses significant antinociceptive and antiinflammatory effects in the
animal models used. The results also suggest that the analgesic effects may be
mediated both centrally as well as peripherally, while the antiinflammatory
activity may be effective in both early and late phases of inflammation. The
results obtained may therefore be used to rationalize the use of the plant in
the treatment of pain and fever in traditional medicine.
Key words: Dennettiatripetala, Essential Oil, Antinociceptive, Anti-inflammatory and
Rodents
Introduction
The fruits of
the plant Dennettia tripetala G.Baker (Annonaceae) are well known in
many communities of some southern states of Nigeria. The plant is commonly
found within cocoa plantation where it is used as means of demarcation of farm
boundaries. The fruits, leaves, bark and roots of the plant possess strong
pepperish and pungent taste. These various parts of the plant are popularly
used as spices and condiments.The plant also possesses characteristic
aroma and fragrances. The fruits are mainly chewed raw in different forms
(fresh green, fresh ripened brown, black dry fruits and dry seeds). The leaves
are commonly used in pepper soup delicacies, and as condiment in some special
local dishes (Ejechi and Akpomedaye, 2005). The leaves are commonly used by the
local herbalists in combination with other medicinal plants to treat various
ailments including fever, infantile convulsion, typhoid, cough, worm
infestation, vomiting, and stomach upset (Oyemitan, 2006). There are also
reports that the fruits are sometimes used for masking mouth odour (Oyemitan,
2006). The fruits of the plants have been reported to be popularly used as
stimulants (Aiyeloja and Bello, 2006; Ndukwu and Nwadibia, 2006; Oyemitan et
al., 2006). Earlier reports showed that the estimated LD50 values of
the oil following oral (p.o.) and intraperitoneal (i.p.) routes in rats were
1,265 mg/kg (p.o.) and 775 mg/kg (i.p.), while the values in mice were 2,150
mg/kg (p.o.) and 470 mg/kg (i.p.) respectively (Oyemitan, 2006). The reported
mechanism(s) of the behavioural effects (novelty-induced and exploratory
behaviours) of the essential oils of the plant have been linked to opioidergic
and GABA-ergic pathways (Oyemitan, 2006). This present study was therefore
carried out to further assess the activities of the essential oil of the plant
for analgesic and antiinflammatory effects in mice and rats respectively as
some of the ethnomedical uses are related to pain-inflammation disorders. The
results of this study may also be used to justify the ethnomedicinal uses of
the plant to treat fever and cough by the people traditionally.
Materials and methods
Plant materials
Fresh fruits were purchased from Owena market, Owena Town, Ondo-East Local Government and Central market, Ondo town, Ondo-West Local
Government Area of Ondo State. All collections were made within the period of
April and May 2005. The fruits of D. tripetala G.Baker (Annonaceae) were
authenticated by Mr. A. Oladele, the Herbarium officer, Department of
Pharmacognosy, Faculty of Pharmacy, and Dr. H.C Illoh of the Department of
Botany, Faculty of Science, Obafemi Awolowo University (OAU) Ile-Ife, Osun
State. The voucher specimen of the leaves and the fruits (from Owena and Ondo) were
prepared and deposited at the Herbarium of the Department of Botany, Faculty of
Science, O.A.U, Ile-Ife, as voucher No. 15,356.
Essential
Oil of D.tripetala:
Distillation of essential oils was carried out using a distillation and
clevenger apparatus. Fresh fruits of D. tripetala were air dried at room
temperature and commuted into coarse powders using pestle and mortar. Four
hundred (400 gram) of the powder was hydrodistilled and it yielded 14.68 g
amounting to 3.7%w/w of the characteristic pungent aromatic odour of the
essential oil. The oils obtained were stored in a lightproof bottle and kept in
a refrigerator until use (Vale et al., 1999; Agbakwuru et al., 1979;Trease and
Evans, 1978). The relative density of the essential oil obtained was
determined using the 10 ml capacity density bottle (British Pharmacopoeia, 1980). The oil was emulsified with 5% Tween 80 shortly
before administration.
Animals
Swissalbino rats (both sexes) weighing 150-200
g andwhite albino mice (both sexes) weighing 18-25 g were obtained from
the Department of Pharmacology, Faculty of Pharmacy, Obafemi Awolowo
University, Ile-Ife, Nigeria. The animals were kept under standard laboratory
conditions and fed with animal feeds (Ladokun feeds, Ibadan, Nigeria) and given water ad libitum prior and throughout the period of experimentation. All
experiments were carried out in accordance with NIH Guide for the care and Use
of Laboratory animals.
Drugs
The
following drugs were also used: Naloxone, Morphine (Sigma, St. Louis, USA), Acetic acid (BDH Chemicals Ltd, Poole, England), Aspirin (Dispirin® ) (Reckitt and Colman, UK), diclofenac (Supreme Pharm. Nig. Ltd., Lagos, Nigeria), indomethacin (methacin ®) (Hovid Bhd, Perak, Malaysi) and formalin (BDH
Chemicals Ltd., Poole, England).
Materials
and Methods
Analgesic
experiment
Hot
plate test:
Twenty
mice were randomly allocated to four groups (n=5). Mice in group 1 were
intraperitoneally (i.p.) administered with 10 ml/kg of 5% Tween 80; mice in
groups 2-4 were intraperitoneally administered with 12.5, 25.0 and 50.0 mg/kg
of essential oil. Each mouse was dropped gently on the hot plate maintained at 55.0
± 0.5 OC and the time taken for the mouse to lick the paw was
recorded at time 0 second (before treatment) and at time 30, 60, 90 and 120
minutes after treatment. The cut off time was set at 15 s to avoid tissue
damage. In another sets of experiment, 4 groups containing 5 mice each were
randomly selected. Group 1 was administered with subcutaneous (s.c) injection
of naloxone (2 mg/kg) and tested as above, groups 2 and 3 were pretreated
with naloxone (2 mg/kg, s.c) 15 minutes prior to administration of 25.0 or 50.0
mg/kg oil, while group 4 was pretreated with subcutaneous administration of
naloxone (2 mg/kg, s.c.) 15 minutes prior to morphine (10 mg/kg, i.p.) and the
mice were tested as earlier described (Viana et al., 2000; Silva et al., 2003).
Acetic
Acid-Induced writhings in mice
Four groups of 5 mice each were randomly selected
(n=5). Mice in group 1 were intraperitoneally administered with 5% Tween 80 (10
ml/kg), while mice in groups 2-4 were administered with 12.5, 25.0 and 50 mg/kg
of essential oil respectively. Thirty minutes after treatment, each mouse was
administered 10 ml/kg of 1% acetic acid (i.p.) and allowed 5 mins delay before
assessment for up to 20 mins inside the Plexiglas’s cage(25 cm x 25 cm
x 30 cm). The number of writhings displayed by each mouse was counted and
recorded. Aspirin (100 mg/kg, i.p.) and morphine (10 mg/kg, i.p.) were
administered to another groups of mice (n=5) that were used as positive
controls. In another experiment, two groups of 5 mice each were pretreated with
naloxone (2 mg/kg, s.c.) 15 minutes prior to administration of the essential oil
(25.0 mg/kg, i.p.) or morphine (10 mg/kg, i.p.). After 30 minutes 1% acetic acid
(10 ml/kg, i.p) was administered and the number of writhes displayed by the
mice were counted and recorded as earlier described above for 15 mins period (N’gouemo
et al., 1996; Le-Bars et al., 2001; Yin et al., 2003).
Formalin
Test
The
method used was that described by Elisabetsky et al. (1995) and Hunskaar and
Hole (1997) with little modification. Seven groups of mice consisting of 5 mice
each were randomly selected. Mice in group 1 (control) was administered with 5%
Tween 80 (10 ml/kg i.p.), while mice in groups 2-4 were treated with the
essential oil (12.5, 25.0 and 50.0 mg/kg, i.p.). Mice in groups 5-7 were
treated with morphine (10 mg/kg i.p.), diclofenac (5.64 mg/kg i.p.) and
Indomethacin (80 mg/kg, i.p.) respectively 30 minutes prior to administration
of 0.02 ml of 2.5% formalin into the sub-planter space of the right hind paw
and the duration of paw licking was determined 0-5 minutes (1st
Phase or neurogenic phase) and 20-25 mins (2nd phase or inflammatory
phase) after formalin administration. The 1st phase is regarded as
the neurogenic mechanism and the 2nd phase is the inflammatory
mechanism (Elisabetsky et al., 1995; Hunskaar and Hole, 1997; Yin et al., 2003).
In another experiment, 3 groups of mice consisting of 5 mice each were selected
and pretreated with naloxone (2 mg/kg, s.c.) 15 minutes prior to administration
of the oil (25 and 50 mg/kg i.p.) and morphine (10 mg/kg i.p.) respectively.
Thirty mins later, they were treated with 2.5% formalin and assessed as earlier
described above.
Antiinflammatory experiment
Carrageenin-induced
paw oedema in rats
The antiinflammatory activity was studied using
carrageenin-induced paw oedema (acute inflammation) method in rats (Winters et
al., 1962). Twenty-five rats were randomly divided into five groups (n=5). Rats
in group 1 (control) were intraperitoneally (i.p.) administered with 5% Tween
80 (10 mg/kg, i.p.), while rats in group 2-4 were intraperitoneally administered
with the 12.5, 25.0 and 50.0 mg/kg of essential oil and rats in group 5 were
intraperitoneally administered with dexamethasone (10 mg/kg, i.p.). Thirty
minutes later, 1% carrageenin (0.1 ml) was injected into the sub-planter
surface of right hind paw of each of all the rats in all the groups.
Measurement of paw size was done by wrapping a piece of cotton thread round the
treated paw of each rat and measuring the circumference on a meter rule (Olajide
et al., 2000; Yin et al., 2003). The measurement was carried out at time 0, 1,
2, 3, 4 and 5 h respectively. Inhibitory activity was calculated at 1, 2 and 3
h after carrageenan treatment (representing the peaks of oedema size), using
the formula:
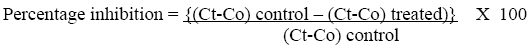
Where
Ct is paw size after a specific time interval in hours after carrageenin
injection and Co is paw size before carrageenin injection.
Statistical
analysis
The results obtained were presented as means ± SEM and
analyzed using analysis of variance (ANOVA) followed by Dunnet test. The level
of significance was set at 95%, p<0.05 for all treatment carried out
compared to control group using the Primer of Biostatistics by Stanton A.Glantz
(version 3.01) copyright (C) 1992 by Mc Graw-Hill Inc.
Results
Analgesic
effects of oil in mice
Hot-plate
Test:
The essential oil at all the dose levels used induced
significant (p< 0.05) analgesia in mice by causing an increase in the
reaction time to thermal stimulus of the hot plate. Pretreatment with naloxone
(2 mg/kg) reversed the analgesia induced by the oil at these dose levels of
25.0 and 50.0 mg/kg significantly at all the time intervals of assessment
(Table 1).
Acetic
Acid-Induced Writhings in Mice
The essential oil dose-dependently inhibited acetic
acid-induced writhes in mice significantly (p<0.05) compared to control, but
the percentage protection was significantly (p<0.05) less than the standard
analgesic used, aspirin (Table 2).Naloxone (2 mg/kg) alone did not
inhibit acetic acid-induced writhings in mice. Pretreatment of mice with
naloxone (2 mg/kg) prior to administration of the oil blocked the inhibitory
effects of the oil on acetic acid-induced writhings in the mice.
Formalin
test
The
essential oil at 12.5, 25.0 and 50 mg/kg (i.p.) dose dependently inhibited
paw-licking time at the two phases compared to control. Indomethacin (80
mg/kg, i.p.) a potent non-steroidal anti-inflammatory drug (NSAID), diclofenac
(5.46 mg/kg, i.p.) another potent NSAID and morphine (10 mg/kg, i.p.) a potent
opioid also showed antinociception. The essential oil at the dose of 50 mg/kg
(i.p.) showed 100% inhibition in the 2nd phase as obtained with
standard drugs used such as morphine (10 mg/kg), diclofenac (5.46 mg/kg) and
the oil (50 mg/kg). Pretreatment with naloxone (2 mg/kg, s.c.) 15 minutes prior
to injection of morphine (10 mg/kg) and
oil (25.0 and 50.0 mg/kg) significantly reduced their antinociceptive effects
at the two phases (Table 3)
The
results of anti-inflammatory effects of the oil in rats.
The oil at the entire dose levels used inhibited
significantly (p<0.05) carrageenan-induced rat paw oedema dose-dependently
compared to the control. Dexamethasone showed greater inhibition than the oil
(12.5-50 mg/kg) at 1h and 2 h but lower inhibition than the oil (25 or 50
mg/kg) at 3h (Table 4).
Table 1:The
antinociceptive activity of the essential oil, morphine and their antagonism by
naloxone assessed by the hot-plate test.
|
Treatment
(N=5
per group) |
Reaction
Time (Second) |
|
T.0
min |
T.30
min |
T.60
min |
T.90
min |
|
Control
(10 ml/kg 5% Tween 80) |
15.00
± 0.55 |
13.80
± 0.58 |
13.20
± 0.55 |
11.50
± 0.56 |
|
EO
12.5 mg/kg |
14.10
± 0.77 |
13.10
± 0.66 |
18.20
± 0.71* |
18.70
± 1.11* |
|
EO
25.0 mg/kg |
13.20
± 0.71 |
22.40
± 0.67* |
22.60
± 0.73* |
17.20
± 0.59* |
|
EO
50.0 mg/kg |
12.50
± 0.50 |
17.70
± 0.76* |
19.10
± 0.81* |
18.50
± 0.76* |
|
NAL
2 mg/kg + EO 25.0 mg/kg |
14.30
± 0.54 |
11.40
± 0.58** |
9.30 ± 0.59** |
10.40
± 0.76** |
|
NAL
2 mg/kg + EO 50.0 mg/kg |
13.70
± 0.75 |
11.76
± 0.66** |
10.80 ± 0.64** |
9.40
± 0.62** |
|
MPH
10 mg/kg |
12.40
± 0.53 |
16.90
± 0.48* |
19.30
± 0.45* |
22.90
± 0.65* |
|
MPH 10 mg/kg + NAL 2 mg/kg |
12.80
± 0.55 |
11.58
± 0.46# |
11.60
± 0.58# |
12.20
± 0.50# |
Each
value is mean ± S.E.M, n=5. EO is essential oil of D. tripetala,
NAL is naloxone and MPH is morphine. The EO (12.5, 25.0 and 50 mg/kg, i.p) and
morphine (10 mg/kg, i.p) showed significant antinociceptive effect when
compared to control group. However, their antinociceptive actions were blocked
by pretreatment with naloxone (2 mg/kg, s.c.) 30 minutes prior to injection of
morphine (10 mg/kg, i.p.) or oil (25 or 50.0 mg/kg, i.p.). * P < 0.05
compared with control; **p<0.05 compared with corresponding dose of EO
alone; #p<0.05 compared with morphine alone.
Table 2. The
antinociceptive activity of the essential oil and morphine and their antagonism
by naloxone assessed by Acetic Acid-Induced Writhings in mice
|
Treatment
(n
= 5 for each group)
|
Number
of writhes (mean ± SEM) |
Percentage
Analgesia |
|
Control
(5% Tween 80) 10 ml/kg |
53.40
± 1.09 |
- |
|
EO
(12.5 mg/kg) |
37.60
± 0.93* |
29.6% |
|
EO
(25.0 mg/kg) |
16.60
± 1.31* |
64.4% |
|
EO
(50.0 mg/kg) |
19.00
± 0.42* |
68.9% |
|
Acetylsalicylic
acid (ASA) (100 mg/kg) |
13.90
± 1.43* |
74.0% |
|
NAL
(2 mg/kg) |
55.00 ± 1.02 |
-3.0% |
|
NAL
(2 mg/kg) + EO (25.0 mg/kg) |
55.80 ± 0.88** |
0.7% |
|
MPH
(10 mg/kg) |
40.60 ± 0.65* |
24.0% |
|
MPH (10 mg/kg) + NAL (2 mg/kg) |
51.20 ± 1.27# |
4.1% |
Each value is mean ± S.E.M, n =5. EO is
essential oil of D. tripetala, NAL is naloxone, and MPH is morphine. The
essential oil at 12.5, 25.0 and 50 mg/kg, i.p. dose dependently reduced acetic
acid-induced writhes in mice (29.6, 64.4 and 68.9% respectively) compared to
control but aspirin (NSAID) at 100 mg/kg, i.p. showed highest analgesic
activity (74.0%). Pretreatment with naloxone (opioid antagonist) completely
blocked the antinociceptive effects of EO at the selected dose of 25 mg/kg and
also reversed that of morphine. *P < 0.05 compared with control, **p<0.05
compared with EO at 25 mg/kg and #p<0.05 compared with morphine alone.
Table 3: The
antinociceptive activity of the essential oil and morphine and their antagonism
by naloxone assessed by the formalin test in mice.
|
Treatment
(N=5
mice for each group)
|
Licking
time in seconds |
Percentage
inhibition |
|
1st
phase |
2nd
phase |
1st
phase |
2nd
phase |
|
Control
(10 mg/kg 5% Tween 80) |
124.2
± 1.0 |
32.6
± 1.0 |
- |
- |
|
EO
(12.5 mg/kg) |
104.6
± 1.5* |
25.0
± 0.9* |
15.8 |
23.3 |
|
EO
(25 mg/kg) |
92.0
± 0.9* |
10.0
± 0.8* |
26.0 |
69.3 |
|
EO
(50.0 mg/kg) |
65.2
± 1.8* |
0* |
47.5 |
100 |
|
Indomethacin
80 mg/kg |
90.2
± 1.2* |
18.4
± 1.3* |
27.4 |
43.6 |
|
MPH
(10 mg/kg) |
18.2
± 1.2* |
0* |
85.3 |
100 |
|
Diclofenac
(5.46 mg/kg) |
97.2
± 1.4* |
0* |
22.7 |
100 |
|
NAL
(2 mg/kg) + EO (25 mg/kg) |
114.6
±1.4** |
17.8
± 0.9** |
7.7 |
45.4 |
|
NAL
(2 mg/kg) + EO (50 mg/kg) |
68.8
± 1.3 |
0* |
43.5 |
100 |
|
NAL
(2 mg/kg) + MPH (10 mg/kg) |
133.2
± 1.3# |
36.0
± 0.9# |
- |
- |
Each value is mean ± S.E.M, n=5. EO is
essential oil of D. tripetala, NAL is naloxone, and MPH is morphine. The
essential oil at 12.5, 25.0 and 50 mg/kg i.p. dose dependently inhibited
paw-licking time at the two phases compared to control. Indomethacin 80 mg/kg,
i.p. and diclofenac 5.46 mg/kg, i.p. are NSAID agents and morphine (a potent
opioid agonist) 10 mg/kg, i.p. also showed antinociception. Morphine (10
mg/kg), diclofenac (5.46 mg/kg) and the oil (50 mg/kg) showed 100% inhibition
in the 2nd phase. Pretreatment with naloxone 2 mg/kg s.c. 15 minutes prior to injection of morphine (10 mg/kg) and EO (25.0 and 50.0 mg/kg) significantly
reduced their antinociceptive effects at the two phases except the oil at 50
mg/kg, which was not affected at the 2nd phase. * P <
0.05 compared with control; **p<0.05 compared with EO at 25 mg/kg and
#p<0.05 compared with morphine alone.
Table 4: The antiinflammatory effects of the essential oil
using the carrageenan-induced paw oedema test in rats.
|
Treatment
(n=5) |
Oedema size (mm) and percentage inhibition of paw
oedema over a period of time intervals (hrs) |
|
Oedema
size in mm (%) 0 h |
Oedema
size in mm (%) 1h |
Oedema
size in mm (%) 2 h |
Oedema
size in mm (%) 3 h |
|
Control
5%Tween
80 (10 ml/kg)
|
24.8
± 0.4 |
37.0
± 0.6 |
40.0
± 0.7 |
39.6
± 0.5 |
|
EO
(12.5
mg/kg)
|
23.2
± 0.4 |
30.4
± 0.7
(40.0%)
|
34.2
± 0.6
(28.0%)
|
31.0
± 0.5
(47.0%)
|
|
EO
(25
mg/kg)
|
24.6
± 0.4 |
26.8
± 0.4
(84.0%)
|
31.6
± 0.6
(54.0%)
|
29.6
± 0.6
(66.0%)
|
|
EO
(50
mg/kg)
|
22.2
± 0.5 |
25.4
± 0.5
(73.8%)
|
26.4
± 0.5
(72.0%)
|
26.8
± 0.7
(69.0%)
|
|
Dexamethasone
(1
mg/kg)
|
24.4
± 0.5 |
25.6
± 0.7
(90.0%)
|
27.5
± 0.7
(75.0%)
|
29.3
± 0.5
(60.0%)
|
EO
is the essential oil of D.tripetala. The oil at the entire dose levels
used inhibited significantly (p<0.05) carrageenan-induced rat paw oedema
dose-dependently compared to the control. Dexamethasone showed greater
inhibition than the oil (12.5-50 mg/kg) at 1h and 2 h but lower inhibition than
the oil (25 or 50 mg/kg) at 3h. *p<0.05 compared with control.
Discussion
The hot plate method is very effective for evaluating
drugs possessing analgesic property, which act centrally (Vale et al., 1999;
Haque et al., 2001; Silva et al., 2003; Al-Naggar et al., 2003).
Prolongation of reaction time in hot plate test inferred possible central
analgesic effects of the oil. The oil increased the reaction time significantly
at the dose levels used compared to control group. Acetic Acid-induced writhing
has been used to evaluate drugs possessing peripheral analgesic effects (Koster
et al., 1959; Viana et al., 2000). In this study, the oil exhibited this
analgesic effect in mice by inhibiting the acetic acid induced writhes, which
is a model of visceral pain, however, not as much as that of the standard drug
used-acetylsalicyclic acid (ASA). Acetic acid has been reported to cause
hyperalgesia by liberating endogenous substances such as prostaglandins,
leukotrieines, 5-HT, histamine, kinins, H+ and K+, etc.
which have been implicated in the mediation of pain perception (Forth et al.,
1986; Rang et al., 1999). Therefore, in an attempt to understand the possible
mechanism of the observed analgesic action of the oil in the hot plate
experiment, mice were pre-treated with naloxone, a potent opioid antagonist
prior to administration of the oil (Almeida et al., 2003).
From the results obtained, it was observed that naloxone blocked both hot plate
and acetic acid induced writhings antinociceptive effects. Naloxone reversed
the analgesic effects of the oil at the two highest dose levels chosen (Table
2). These results suggest that the oil may be exhibiting its analgesic effects
in similar manner to opioids or opiates. The antagonistic effects of naloxone
on the analgesic effects of the oil persist even beyond 90 minutes assessment
period therefore suggesting that the analgesic effect of the oil as
demonstrated is probably mediated through the opioid system in the central nervous
system (CNS). This is in line with the previous reports that opioid receptors
are also involved in peripheral as well as central (μ and ĸ-opioids
and monoamines) mechanisms of analgesia in animals (Millan et al., 1994).
Prolongation of reaction time in hot plate test confirmed central analgesic
action that was blocked by the opioid antagonist, naloxone, a specific
antagonist of opioidmimetic receptors (Almeida et al., 2003).
Yin et al (2003) reported that many studies have shown
that the earlier phase (1st phase) of formalin-induced pain reflects
the direct effect of formalin on nociceptors whereas the late phase (2nd
phase) reflects inflammatory pain, which has been linked to prostaglandin
synthesis (Hong and Abbot, 1995; Yin, et al., 2003). Opioid
analgesics have been reported to possess antinociceptive effects in both phases
having more effect at the 2nd phase (Le Bars et al., 2001).
Non-steroidal anti-inflammatory drugs (NSAIDS) such as indomethacin is said to
be effective only in the 1st phase especially if the formalin is
injected at high concentration (Yashpal and Coderre, 1998). In this study, the
oil dose-dependently inhibited nociception induced in the Formalin Test
significantly compared to control group in the 1st phase
(neurogenic) and 2nd phase (inflammatory). These results therefore
further suggest that the oil contain constituents that exhibit
anti-inflammatory properties. Commonly used Non-Steroidal anti-inflammatory
Drugs (NSAID) such as aspirin and indomethacin are widely used to reduce
swelling associated with pain and inflammation through inhibition of
prostaglandin synthesis by direct effect on cyclo-oxygenase (COX) in the
arachidonic acid (AA) metabolism (Amos et al., 2001; Nwafor
and Okwuasaba, 2003). This result showed that the oil possesses an
anti-inflammatory effect dose-dependently in the 1st phase including
the standard drugs such as indomethacin and diclofenac when compared to control
mice. Morphine, a potent opioid agonist inhibited paw licking (85%) inhibition,
which was completely blocked by the opioid antagonist, naloxone. However,
pretreatment with naloxone prior to administration of the oil did not block the
analgesic effects in the 1st phase at a dose of 50 mg/kg, i.p. but
blocked this effect with administration of the oil at 25 mg/kg, i.p. This
result suggests that the oil contain constituents that have potent analgesic effects
that may be acting in a similar manner to opioids. In the 2nd phase,
there was a reduction in paw licking compared to control. In this phase,
pretreatment with naloxone had no effect on the analgesic effect of the oil at
this dose level. The results showed that the antagonist only had effect on the
low dose but had no effect on the high dose of the oil administered. When the
oil (50 mg/kg) was assessed, morphine and diclofenac completely blocked paw
licking compared to control in the 2nd phase whereas indomethacin
has lower effects at this phase. Naloxone (2 mg/kg), only reversed the
analgesic effect of the oil at the dose of 25 mg/kg, while it has no effect on
morphine and oil (50 mg/kg, i.p.) suggesting that both naloxone and the oils
probably compete for the same receptor sites-opioidergic receptor and further
suggests that opioid receptors are involved in the mechanism of action. This
result showed that the oil has analgesic effects at both phases of the formalin
induced paw-licking episodes in mice. This result also suggests that there may
be central mediation of the anti-inflammatory effect of the oil because of its
effects in the 2nd phase. There is no doubt that morphine exerts its
main analgesic effects centrally, hence it appears to be more effective at the
two phases (Viana et al., 2000). The results further showed that the oil has a
comparable effects with the standard drugs used.
Carrageenan-induced paw oedema as an in vivo model of
inflammation has been extensively used to evaluate the antiedematous effects of
natural products. The EO of D. tripetala at all the dose levels used
dose-dependently displayed significant (p<0.05) anti-inflammatory effects by
inhibiting the carrageenan-induced paw oedema in the rats compared to control
(Table 4). This result is not unusual as essential oils from other sources such
as from Eucalyptus sp. (Silva et al., 2003), radix of
Asarum sieboldii (Kim et al., 2003) and Lavandula
angustifolia (Hajhshemi et al., 2003) among several reports
demonstrated significantly, strong analgesic and anti-inflammatory properties.
The results obtained in this study indicated that the EO of D tripetala may
possess more lasting or prolonged anti-inflammatory effects than the standard
steroidal drugs as the EO (25-50 mg/kg) inhibited the paw oedema at 3 hr more
significantly than dexamethasone (1 mg/kg). However, these results are
preliminary and therefore other inflammation models will be explored to further
validate this anti-inflammatory property of EO and determine probable mechanism(s)
involved. The EO of D.tripetala has been reported to contain mainly
β-phenylnitroethane (80%), l-linalool (11%), β-Eudesmol and nerolidol
(4%) (Agbakwuru et al., 1995). Therefore, it could be suggested that the
antinociceptive and anti-inflammatory effects demonstrated by the EO may be due
to one or more of these compounds. Further works are however underway to carry
out activity-directed fractionation of the EO in order to determine the active
compound(s) responsible for its effects. The various analgesic effects obtained
in this work provide lead for detailed and comprehensive knowledge into Dennettia
tripetala as a potential candidate for development into potent patentable
analgesic drug.Combining the results from hot plate, acetic acid-induced
writhes and formalin tests suggest that the oil contains constituent(s) with
potent analgesic effects that are likely to be mediated both peripherally and
centrally.
In conclusion, the results of the analgesic and
antiinflammatory properties of the essential oil of D.tripetala further
justify the use of the plant in ethnomedicine for treating fever, cough and
vomiting and there is need for further investigation with isolated components
of the oil for possible development into new class of analgesic and antiinflammatory
drugs.
References
- Agbakwuru E.O.P, Osisiogu I.U, and Rucker
G. (1979). Constituents of essential oil of Dennettia tripetala G.Baker
(Annonaceae). Nig. J. Pharm., 10: 203-208.
- Aiyeloja A.A. and Bello
O.A. (2006). Ethnobotanical potentials of
common herbs in Nigeria; A case study of Enugu State. Educational Res. Review, 1: 16-22.
- Almeida E.R., Almeida
R.N., Navarro D.S., Bhaitacharryya J., Silva B.A. and Birnbaum J. (2003). Central anti nociceptive effect of a hydro-alcoholic
extract of Dioclea grandiflora seeds in rodents. J. Ethnopharmacol., 88: 1-4.
- Al-Naggar T.B.,
Gomez- Serranillos M.F., Carretero M.E. and Villar A.M. (2003) Neuropharmacological activity of Nigella sativa L. extracts. J. Neuropharmacol., 88: 63-68
- Amos S., Adzu B., Binda
L., Wambebe C. and Gamaniel K. (2001). Neuropharmacological
effects of aqueous extract of Sphaeranthus senegalensis in mice. J.
Ethnopharmacol., 78: 33-37.
- British Pharmacopoeia Vol II
(1980) London His Royal Majesty’s Stationary pA77.
- Ejechi B.O. and Akpomedaye D.
(2005). Activity of essential oil and phenolic extract of pepper fruits, Dennettia
tripetala G. Baker against some food-born microorganisms. Afr. J. Biotechnol., 4(3): 258-261.
- Elisabetsky E.,
Amador T.A., Albuquerque R.R., Nunes D.S, and Carvalho A.C.T. (1995). Analgesic activity of Psychotri colorata (Wild
ex R and S) Muell Arg. Alkaloids. J. Ethnopharmacol.,148: 77-83.
- Forth W, Martin . and, Peter K. (1986). The relief of pain.
Hoechst medication Up-Date. Hoechst, Munich, pp. 6-107.
- Hajhashemi J.,
Alireza-Ghannadi and Badies Sharif (2003). Anti-inflammatory
and analgesic properties of the leaf extract and essential oil of Lavandula
angustifolia Mill. J. Ethnopharmacol., 89: 67-71.
- Haque S., Choudhuri M.S.K.,
Islam M.N., Hannan J.M.A. and Shahriar M. (2001). Pharmacological study of Sri
Mahalaxmi Bilas (Rasayan). Hamdard Medicus, 44: 54-60
- Hong Y. and Abbot F.V. (1995).
Peripheral opioid modulation of pain and inflammation in the formalin test. Eur.J.
Pharmacol., 227: 21-28.
- Hunskaar S. and Hole K. (1997).
The formalin test in mice. Dissociation between inflammatory and
non-inflammatory and pain. Pain, 30: 103-114.
- Kim Sung-Jin, Zhang Cheng Gao, and
Lim Jung Taek. (2003). Mechanism of antinociceptive effects of Asarum sieboldii Miq. Radix; Potential role of bradykinin, histamine and opioid
receptor-mediated pathways. J. Ethnopharmacol., 88: 5-9.
- Koster R., Anderson M. and
De-Beer E.J. (1959).Acetic-Acid-Induced analgesic screening. Federation
Proceedings, 18: 412.
- Le-Bars D., Gozariu M. and
Cadden S.W. (2001). Animal Models of Nociception. Pharmacol. Review, 53: 597-652.
- Millan M.J. (1994) Serotonin
and Pain; evidence that activation of 5-HT-1A receptors does not elicit
anti-nociception against noxious thermal, mechanical and chemical stimuli in
mice. Pain, 58: 45-61.
- N’gouemo P., Baldy-Moulinier M.
and Nguemby-Bina C. (1996). Effects of ethanolic extract of Desmodium
adscendens on central nervous system in rodents. J. Ethnopharmacol., 52: 77-83.
- Ndukwu B.C. and Nwadibia N.B.
(2006). Ethnomedical Aspects of Plants Used as Spices and condiments in the
Niger-Delta Area of Nigeria. http//www.siu.edn/n eb/leaflets/niger.htm..
- Nwafor P.A. and
Okwuasaba F.K. (2003). Antinociceptive and
anti-inflammmatory effects of methanolic extract of Asparagus pubescens root in rodents. J. Ethnopharmacol., 84: 125-129.
- Olajide A.O., Awe S.O.,
Makinde J.M., Ekhelar A.I., Olusola A., Morebise O. and Okpako D.T. (2000). Studies on the anti-inflammatory, antipyretic and
analgesic properties of Alstonia boonei stern bark. J.
Ethnopharmacol., 71: 179-186.
- Oyemitan I.A., Iwalewa E.O., Akanmu
M.A., Asa S.O. and Olugbade T.A. (2006). The Abusive Potential of Habitual
Consumption of the Fruits of Dennettia tripetala G.Baker (Annonaceae)
Among the People in Ondo Township (Nigeria). Nig. J. Natural Products Med., 10: 55-62.
-
Oyemitan I.A. (2006). Evaluation of
Dennettia tripetala G. Baker (Annonaceae) for Central Nervous System
Activities. An M.Phil Thesis. Department of Pharmacology, Obafemi Awolowo University, Ile-Ife, Nigeria.
-
Rang H.P., Dale M.M. and Ritter
J.M. (1999). Pharmacology. Churchill Livingstone, 5th edn.
- Silva J., Abebe W.,
Sonsa S.M., Duarte V.G., Machado M.I.L. and Matos FJ..A. (2003)..Analgesic and antiinflammatory effects of essential oil
of Eucalyptus. J. Ethnopharmacol., 89: 277-283.
-
Trease G.E. and Evans W.C. (1978).
Trease and Evans Pharmacognosy. Bailliere Tindall Ltd, London, 11:
405-474.
- Vale T.G., Matos
F.J.A., de-Lima T.C.M. and Viana G.S.B. (1999). Behavioural effects of essential oils from Lippia
alba (Mill) N.E Brown Chemotypes. J. Ethnopharmacol., 167: 127-133.
- Viana G.S.B., Vale
T.G., Pinho R.S.N. and Matos F.J.A. (2000).
Anti-nociceptive effect of the essential oil from Cymbopogon citratus in
mice. J. Ethnopharmacol., 70: 323-327.
- Winters C.A., Risley E.A. and Nuss G.W. (1962). Carrageenin induced edema in hind-paw of the rat
as an assay for inflammatory drugs. Proceedings of the Soc. Exptl Biol.
Med., 111: 544-547.
- Yashpal K.. and Coderre
T.J. (1998). Influence of Formalin
concentration on the antinociceptive effects of anti-inflammatory drugs in the
formalin test in rats; Separate mechanism of underlying the nociceptive effects
of low and high concentration formalin. European J. Pain, 2: 63-68.
- Yin Wu, Wang Tian-Shan, Yin
Fang-Zhou and Cai Bao-Chang (2003). Analgesic and anti-inflammatory properties
of brucine and brucine-N extracted from seeds of Strychnos nux-vomica.
J. Ethnopharmacol., 88: 205-214.
© Copyright 2008 - African. Journal. Traditional, Complementary and Alternative Medicines
|
