
|
The Journal of Food Technology in Africa
Innovative Institutional Communications
ISSN: 1028-6098
Vol. 9, Num. 1, 2004, pp. 17-22
|
The Journal of Food Technology in Africa Vol. 9 No. 1, 2004, pp. 17-22
Drying Kinetics, Physico-chemical and Nutritional Characteristics
of "Kindimu",
a Fermented Milk-Based-Sorghum-Flour
*Tatsadjieu N.L.1, Etoa F-X2, Mbofung C.M.F.1
1University of Ngaoundere, School of Agro-Industrial Sciences,
Department
of Food Sciences and Nutrition, Ngaoundere - Cameroon. P.O Box 454.
2University of Yaounde, Faculty of Science, Department of Biochemistry,
Yaounde - Cameroon. P.O Box 812.
*Corresponding author
Code Number: ft04003
ABSTRACT
"Kindimu", a fermented milk-based cereal foods made by sun drying a mixture
of fermented milk and cereal
flour is a common flour ingredient in the central African region. A study was
carried out to evaluate the
effect of processing methods on the drying behaviour, functional and nutritional
quality of such a food
prepared from sorghum flour and fermented milk. A mixture of 1 part sorghum
flour (germinated or non -
germinated) and the 2 part (w/w) on fermented milk was coated on aluminium
trays to a depth of 5mm and
dried at 50, 65 or 80°C. Results obtained indicated that a simple mass
transfer equation Ln [(C-C*)/(Co-C*)]=
-(K/L)t can be used to model the drying behaviour of the fermented milk -sorghum
flour mixtures. The
magnitude of mass transfer coefficient K, increased with drying temperature
and the germination of sorghum.
Germination and addition of milk increased the in vitro protein digestibility
of sorghum flour by 19.03%,
protein solubility by 11.5% and available lysine content by an average of 3.04%
and reduced the phytate
content by 30%. The water absorption capacity of flours was equally reduced
by an average of 4%.
Key words: Fermented milk, sorghum, malting, drying kinetic, physico-chemical
properties, nutritional properties.
INTRODUCTION
Fermentation is one of the oldest
methods of producing and preserving
foods. In Africa, this technology plays a
very important role in the nutrition and
daily diets of many populations.. The
common characteristics of such
fermentation is the production of
organic acids resulting in a reduction of
the pH and a typical sour taste. Besides
the beneficial effects of shelf life
extension and improvement of sensory
properties, it has been shown that certain
fermented milk based products have a
therapeutic effect in diarrhoea disease
(Boudraa et al.,1989).
Fermentation and germination are two
classical technologies commonly used to
improve on the protein digestibility and
the B6 vitamin content of cereals, either
by decreasing the amount of inhibitors
or releasing the nutrients for absorption
(Dhankher and Chauhan, 1987a;
Dhankher and Chauhan,1987b). By
using a lactic fermentation technique
combined with flour of germinated
seeds, the energy density in cereal based
gruel is significantly increased (Svanberg
and Sandberg, 1989), while at the same time bulk density is reduced (Mosha, and
Svanberg, 1990; Marero,et al., 1988).
Germination of cereals has equally been
found to increase the protein quality of
the malted flours apart from modifying
the starch granules (Opoku et al., 1983).
Germination studies have also shown
increase in vitamins and bioavailability
of traces minerals in the cereals sprouts.
In the central African region, and
especially in the northern parts of
Cameroon, cattle rearing and milk
production is the principal
preoccupation of the people. Excess milk
produced is often fermented and mixed
into a dough with cereal flour (maize,
sorghum, millet or rice) which is
subsequently cooked and consumed in
the form of gruel. This traditional
method of processing milk and cereals
permits the utilisation of excess milk and
contributes to ensure food security in
these areas. It certainly also improves
the protein-energy value of cereal based
diets. However the method is laborious,
very susceptible to bacterial
contamination and very likely to
influence the functional properties of
the flours obtained.
As part of main study aimed on
reducing the production time and
improving on the hygienic quality of this
milk-based cereal product, the present
study was carried out to determine the
effect of such production parameters
as germination and drying temperature
on the drying kinetic, the physicochemical
and nutritional properties of
the fermented milk-based-sorghum
flours.
MATERIAL AND METHODS
Production of sorghum flours
A white cultivar of sorghum (Sorghum bicolor), was obtained from the
experimental farm of the Institute for
Agronomic Research (IRAD) in Maroua,
Cameroon. Sorghum was cleaned and
soaked in distilled water (100 g in 300
ml) for 12 hours, rinsed and divided into
2 parts. One part was germinated in the
dark for 72 hours while the second part
was drained dry at ambient temperature
and dried in an oven at 50°C to a
constant weight. The germinated seeds
were equally drained and dried at 50°C.
The different sorghum samples were each milled into a fine flour (150µ)
using
a hammer mill. Flours obtained were
packaged in polyethylene bags and stored
in a fridge at 4°C until needed for use.
Fermentation of milk
Fresh milk was obtained from the
Canadian-run pilot dairy farm
(SOGELAIT) located close to the
University of Ngaoundere - Cameroon.
Pasteurised whole fresh milk was
aseptically inoculated with a dried starter
(Rhône - Poulenc, France) containing a
strain of Streptococcus thermophilus and one
strain of Lactobacillus bulgaricus, followed
by incubation for 3 hours at 40°C in a
thermostatically controlled water bath
and incubated for 3 hours at 40°C.
Production of milk-based sorghum
flours (MBSF)
MBSF were prepared by mixing
fermented milk and cereal flours in a
2:1 ratio (w/w) using a food mixer
(Kenwood, UK) to ensure proper mixing
and allowed to rest for 1 hr. The dough
obtained was spread uniformly on
aluminium plates of dimension 20 x 20
x 0.5 cm and dried to constant weight in
an air drought oven at 50, 65 or 80°C.
The drying process was monitored by
weighing the plates at regular intervals
using a Sartorius balance (sensitivity
0.001g). At the end of the drying
determined by a constant weight, the
dried dough was packaged in
polyethylene bags and stored in a
dessicator at 4°C until needed for use.
Analysis of chemical composition
of flours
MBSF were analysed for moisture,
proteins, ash and lipids essentially
according to standard AOAC methods
of the Association of Official Analytical
Chemists (AOAC, 1975). Flour samples
were acid-hydrolysed and the resulting
reducing sugar designated as available
carbohydrates, was determined by the
Dinitrosalisylic acid (DNS) method of
Fisher and Stein as described by
Fombang (1999). Phytates were
determined by the method described by
Thompson and Erdman (1982).
Analysis of functional properties
of flours
Water absorption capacity (WAC)
The evaluation of the rate of water
uptake of flour was carried out by the Baumann method as described by
Dumay et al. (1986). At least three
measurements were conducted for each
sample and the mean was expressed as
mL of liquid retained per gram of
sample.
Protein water solubility (PWS)
PWS of the MBSF was determined
essentially by the method of Oshodi and
Ekperigin (1989). A sample (0.02g) of
flour was added to 10 ml of distilled
water, mixed with a spatula and
centrifuged at 3500 rpm. The proteins
in the supernatant was determined by
the method of Lowry et al. (1951). The
solubility of proteins in water was
expressed as a percentage of the total
proteins.
Analysis of nutritional properties
of the milky flours.
In vitro protein digestibility was
determined by the method of Savoie
and Gauthier (1986). Following this
method, a sample containing about
450mg of protein was suspended in 17ml
of 0.1 N HCl and incubated in a shaking
water bath for 5 min. The pH was
adjusted to 1.9 and 2.5ml of 0.7%(w/
v) freshly prepared pepsin solution
(Sigma chemical Co, St Louis Mo) was
added, mixed and incubated at 37°C for
30 min. The reaction was stopped by
the addition of 1ml of 1N NaOH and
the volume adjusted to 23ml with
sodium phosphate buffer 0.1M, pH 8.
The lot was then transferred into a
dialysis tubing (Molecular weight cut off~ 1200, Medicell International Ltd,
London, U.K) following by the addition
of 2.5 ml of a phosphate buffer solution
containing pancreatine (0.7%w/v)
(Sigma chemical Co., St Louis Mo). The
tubing was then tied and introduced each
into a beaker containing 400ml of
phosphate buffer. The whole was
incubated at 37°C under agitation. At
regular time intervals, 2ml of dialysate
was withdrawn and the proteins
determined by the methods of Lowry
et al., (1951). The protein digestibility
(PD) at each time was calculated using
the formula:
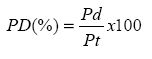
Pd= protein in dialysate and Pt= total
protein in dialysis bag.
Available lysine was determined
according to the method of Kakade and
Liener (1969). Exactly 10.00 mg of
sample was mixed with 1ml of 4%
NaHCO3, pH 8.5 and incubated at 40°C
with agitation for 10min. This followed
by the addition of 1ml of 1% 2,4,6
trinitrobenzenesulfonic acid (TNBS)
further incubation for 2 hours at the end
of which 3ml of concentrated HCl was
added. The tubes were then autoclaved
at 120°C for 1 hr before cooling down
at room temperature. The contents of
the tubes were then diluted with 5 ml
of distilled water, filtered, washed twice
with 5ml of diethyl ether and placed in
boiling water to evaporate traces of
ether. The optical density of the solutions
were read at 346 nm against a blank
treated in the same manner but without
flour. The concentration of lysine was
calculated using the specific absorbance
of e-TNP lysine which is 14600Mcm-1.
RESULTS AND DISCUSSION
Drying behaviour of the flours
The drying curves of the different
MBSF are shown in figure 1. The initial
moisture content of the different milksorghum
mixtures of germinated and
non germinated sorghum were 60.08%
and 59.92 % respectively. Because of
the high level of the initial moisture
content, a constant rate period was
observed during the early parts of the
drying process. The falling rate period
appears directly after the constant rate phase. During this phase, it was assumed
that the resistance to mass transfer of
water vapour from the solid surface to
the air was negligible and the internal
resistance controlled the rate of drying
(Yener et al., 1987).
In this respect, an internal mass transfer
coefficient K was defined as
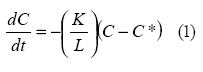
- Where K= mass transfer coefficient, m/
hr,
- L= Thickness of dried material, m,
- t= drying time
- C=Total moisture, kg moisture/kg dry
- solid,
- C*= Equilibrium moisture, kg moisture/kg dry solid
- Integration of equation (1) from Co to
C give
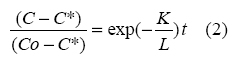
Equation (2) was rearranged as
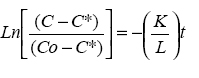
and drying curves were drawn as Ln [(CC*)/(
Co-C*)] versus time (Figure 2).
Slopes of the curve from the beginning
of the falling period were determined
and used to calculate the values of K/
L. Dependence of K/L values on
temperature and germination of
sorghum is shown in table 1. This table
indicate that K/L increased with increase
in drying temperature (P<0.05) and
germination of sorghum grain (P<0.05).
This increase of K/L with germination
of sorghum might be due to the
hydrolysis of the starch and other
hygroscopic macromolecules which
reduce polar bonds capable of binding
to water molecules.
Chemical composition of the
flours
Sorghum flours:
Proximate composition of the sorghum
flours is shown in table 2. Germination
of cereals is known to influence its
phytate content (Larson and Sandberg,
1995). Germination led to a 30%
decrease in phytate content of sorghum.
This level of decrease in phytate is much
lower than that of 79% reported by
Larson and Sandberg (1995) for
germinated barley. The differences in
the rate of reduction obtained in this
study may be due to the difference in
phytase activity in both cereals or to the
different germination time.
The germination of sorghum also results
in a significant (P<0.05) fall in the
proximate composition of sorghum. In
this respect, a decreases in protein, fat
and carbohydrates content were in the
range of 9.9%, 20.8% and 8.2%
respectively. The decrease in fat content
during germination has been reported
by several authors (Kaukovirta et al.,
1993; Dibofori et al.,1994). A reduction
in carbohydrates following germination
has also been reported (Nout, 1991). The
decrease in content of macromolecules
during germination could be due to the
synthesis and activity of hydrolytic enzymes (lipases, proteases amylase).
Germination also led to a 7.67%
decrease in ash content of sorghum. This
decrease in ash content which represents
loss in minerals could be due to the
rootlet and to the washing of the grains
during germination.
Milky-based-sorghum flours
(MBSF)
Proximate composition of the different
MBSF is as shown in table 2. The
composition was not influenced by the
drying temperature. The addition of
fermented milk increased the protein
content in the blend by 43.84% and
45.32% for the flours with non
germinated sorghum and germinated
sorghum respectively. The reducing
sugar levels ranged from 37.7 to 38.9%
for non germinated and 32.4 to 33.6%
for geminated sorghum and reflected the
reducing sugar content of the sorghum
flour. As it would have been expected,
fermented milk also increased the ash
content by 5.17 to 7.24% respectively
for flours with germinated sorghum and
non germinated sorghum. This significant
(P<0.05) increase in ash is due to the
fact that milk is an important source of
such minerals as calcium and
phosphorus. The crude fat content
ranged from 7.61 to 7.83 for non germinated sorghum based flour and
from 7.02 to 7.25 for germinated
sorghum based flour.
Functional properties of the flours
Water absorption capacity and the
protein solubility are important
characteristics of flours because physicochemical
properties such as viscosity and
gelation are dependent on them (Cheftel
et al. 1977).
The initial water absorption rate (Vi)
(measured between 0 to 45 sec), the
maximum absorption capacity (Q) and
the water protein solubility (PWS) of the
milky flours are shown in table 3. Vi
and Q for the flours with non germinated
sorghum were higher than that for
germinated sorghum. Germination of
sorghum led to a reduction in the initial
rate of water absorption and also in the
maximum water absorption capacity.
Depending on drying temperature,
reduction in the initial rate of water
absorption varied between 36.55 and
42.05% while that of maximum water
absorption capacity varied between 2.84
and 4.7%.
On the other hand, germination led to
an increase in PWS with value ranging
from 10.58 to 12.86%. Drying beyond
65°C led to a reduction in PWS. For
flours dried at 80°C, protein solubility
decreased by 14.25 and 15.95% for non
germinated and germinated sorghum
respectively. The observed negative
effect of high drying temperature on the
solubility of proteins may be potentially
due to the formation of complexes
between soluble proteins and sugars such
as is often the case in Maillard reactions.
Nutritional characteristics
The incorporation of fermented milk
into sorghum flour significantly (P>0.05)
improved on its protein content.
Germination of sorghum further
improved on the protein in vitro
digestibility of the protein (Figure 3).
After 180 minutes of in vitro digestion,
flours made from fermented milk and
germinated sorghum showed a
digestibility rate of 23.51% as opposed to that of 19.75% observed for flours
containing non germinated sorghum.
Thus a significant improvement rate of
19.03% was attained in the digestibility
of the flours by germination of
sorghum. This increased rate may
partially be explained by the fact that
during germination, proteolytic enzymes
are synthesized which degrade proteins
to small water soluble peptides (Nout,
1991). Also it may be partially due to the fact that germination led to a felling
in phytate content which is known to
reduce protein digestibility (Poonam and
Sahl, 1994).
Depending on drying temperature, the
addition of milk increased the level of
available lysine in non germinated
sorghum from 18.25 to 40.52 mg/g of
protein while that in germinated
sorghum varied between 21.78 to 40.74
mg/g of protein on the average.
Germination of sorghum increases the
level of available lysine in flour. Increase
in available lysine levels following
germination has also been reported
(Dalby et al., 1976) for wheat. It is
established that during germination the
rate of synthesis of albumins and
glutamins which are proteins rich in lysine
decrease (Okkyung, and Yeshajahu,
1985).
Germination also led to a decrease of
the phytate content of sorghum flour.
In fact fermented milk-based-sorghum
flours are acidic, at this pH range,
proteins are generally charged positively
whereas phytates are negatively charged
(Reddy et al.,1982). As such, phytate
strongly bind protein particularly at their
e-NH2 group of lysine. This attraction
seemed to have been potentiated by high
drying temperatures, specially at 80°C
which a reduction of about 8.05 and
9.03% for germinated and non
germinated sorghum was observed.
CONCLUSION
On the whole, the results of this study
demonstrate the fact that use of
germinated sorghum and appropriate
drying temperature can greatly improve
the chemical and nutritional quality of
the fermented milk-based sorghum flour
commonly called Kindimu. Drying at
temperature below 65°C seem to be
preferable. Consumption of such foods
formulated from locally available food
stuffs may lead to the improvement of
the protein-energy status of the local
population. Several other types of
cereals (maize, sorghum, millet, rice) are
also traditionally used in the production
of these flours. In view of the fact that
these cereals differ in their characteristics
(chemical composition, physico-chemical
properties etc), it would be interesting
to exploit the other cereals for this same purpose to determine which is better
suited to the production of the milkbased
cereal flour.
REFERENCES
- AOAC., 1975. Official Methods of Analysis, 875-
876..Association of Official Analytical Chemists,
Washington.
- Boudraa, G.; Touhami, M.; Pochart, P.; Soltan,
R.; Mary, J.Y and J.F., 1989. Desjeux, Proc..
Congrès International sur Les Laits Fermentés:
Actualité de la recherche, pp. 229-232. Paris
.
- Cheftel, J.C; Cheftel, H and Besançon, P., 1977. Introduction à la biochimie et à la
technologie des aliments, Vol 2 420 pages, Tec. Doc. Lavoisier,
Paris.
- Dalby, A. and Tsai, C.Y. 1976. Lysine and
tryptophane increases during germination of
cereal grains. Cereal Chem., 53 :222-226.
- Dhankher, N. and Chauhan, B.M., 1987a.
Technical note: preparation, acceptability and Bvitamin
content of Rabadi- a fermented pearl
millet food.. Int. J. Food. Sci. Technol 22: 173-176.
- Dhankher, N. and Chauhan, B.M., 1987b. Effect
of temperature and fermentation time on phytic
acid and polyphenol content of Rabadi - a
fermented pearl millet food. J.Food.Sci 52: 489-
490.
- Dibofori, A.N; Okoli, P.N and Onigbinde; A.O.,
1994. Effect of germination on the cyanide and
oligosaccharide content of Lima bean (Phaseolus
linatus). Food chemistry. 51(2) : 133-136.
- Dumay, E.F.; Conder, F. and Cheftel, J.C., 1986. Préparations protéiques de graines de coton glandless: Caractérisation
des constituants
protéiques et propriétés fonctionnelles. Sci.
Aliments, 6: 623 -656.
- Fombang, N. E. , 1999. Studies on the effect
malting conditions on the level of some antinutrients
and nutrients in sorghum and bean
flours.. M.Sc thesis. University of Ngaoundere,
101 pages.
- Kakade, M.L. and Liener, I.E., 1969. Determination of Available
Lysine in Proteins. Analytical Biochemistry. 27: 273-280.
- Kaukovirta, N.A; Laakso, S.; Reinikainen, P.
and Olkku, J., 1993. Lipolytic and oxydative
changes of barley lipids during malting and
mashing. J. of the Institute of Brewing 99 (5): 395-
403.
- Larson, M. and Sandberg, A. S., 1995. Malting
of oats in pilot - plant process. Effects of heat
treatment and soaking conditions on phytate
reduction. J. Cereal Sci. 21 (1) : 87-95.
- Lowry, O.H., Rosebrough, N. J.; Farr, A.L.
and Randall, R. J., 1951. Protein measurement
with the folin phenol reagent. J. Biol. Chemistry,
193 265-269.
- Marero, L.M.; Payumo, E.M., Agninaldo, A.R.
and Homma, A., 1988. Technology of weaning
food fermentation prepared from germination
cereals and legumes. J. Food. Sc. 53 (3) 1391 - 1395.
- Mosha, A.C and Svanberg, U., 1990. Preparation
of weaning foods with high nutrient density using
flour of germinated cereals. Food and Nutrition
Bulletin, 12 69-74.
- Nout, M.J.R., 1991. Weaning foods for tropical
climates. Proc. Regional Workshop on Traditional
African Foods-Quality and Nutrition, pp 23-31.
Dar es Salaam.
- Okkyung, C. and Yeshajahu, P., 1985. Amino- acids in cereal
proteins and protein fractions. In: Digestibility and amino acid availability
in cereals
and oilseed Amino-acids in cereal proteins and
protein fractions. Ed. by Association of Cereals
Chemists, pp. 65-107. Minnosota.
- Opoku, A.R.; Osagie, A.U. and Ekpengin, E.R.,
1983. Changes in the major constituents of millet
(P.americanum) during germination. J. Agric. Food.
Chem. 37 507-509.
- Oshodi, A.A and Ekperigin, M.M.. 1989. Functional properties of
pigeon pea (Cajanus cajan) flour. Food Chem. 34 187-191.
- Poonam, G. and Sahl, S., 1994. Protein and starch
digestibility and iron availability in developed
weaning foods as affected by roasting. J. of Human
Nutrition and Dietetics 7 121-126.
- Reddy, N.R.; Sathe, S.K., and Salunke, D.K.,
1982. Phytates in legumes and cereals. Adv. Food.
Res. 28: 1-92.
- Savoie, L. and Gauthier, F., 1986. Dialysis cell
for the in vitro measurement of protein. J. of Food
Sci. 51 (2) 494-498.
- Svanberg, U and Sandberg, A.S., 1989. Improved
iron availability in weaning foods using
germination and fermentation. In Nutrient
Availability: Chemical and Biological Aspects. Ed.
Southgate DAT, Johnson IT & Fenwick GR. Royal
Soc. Of Chem. Special publication 72: 179-181.
- Thompson, B.D. and Erdman, J.R.W. 1982. Phytic acid determination
in soy beans. J. of Food Sci. 47 513-516.
- Yener, E., Ungan, S. and Özilgen, M., 1987. Drying of Honey-Starch
Mixtures. J. of Food Sci. 52 (4) 1054-1058.
Copyright 2004 - The Journal of Food Technology in Africa, Nairobi
The following images related to this document are available:
Photo images
[ft04003t3.jpg]
[ft04003f2.jpg]
[ft04003f1.jpg]
[ft04003f3.jpg]
[ft04003t4.jpg]
[ft04003t2.jpg]
[ft04003t1.jpg]
|
